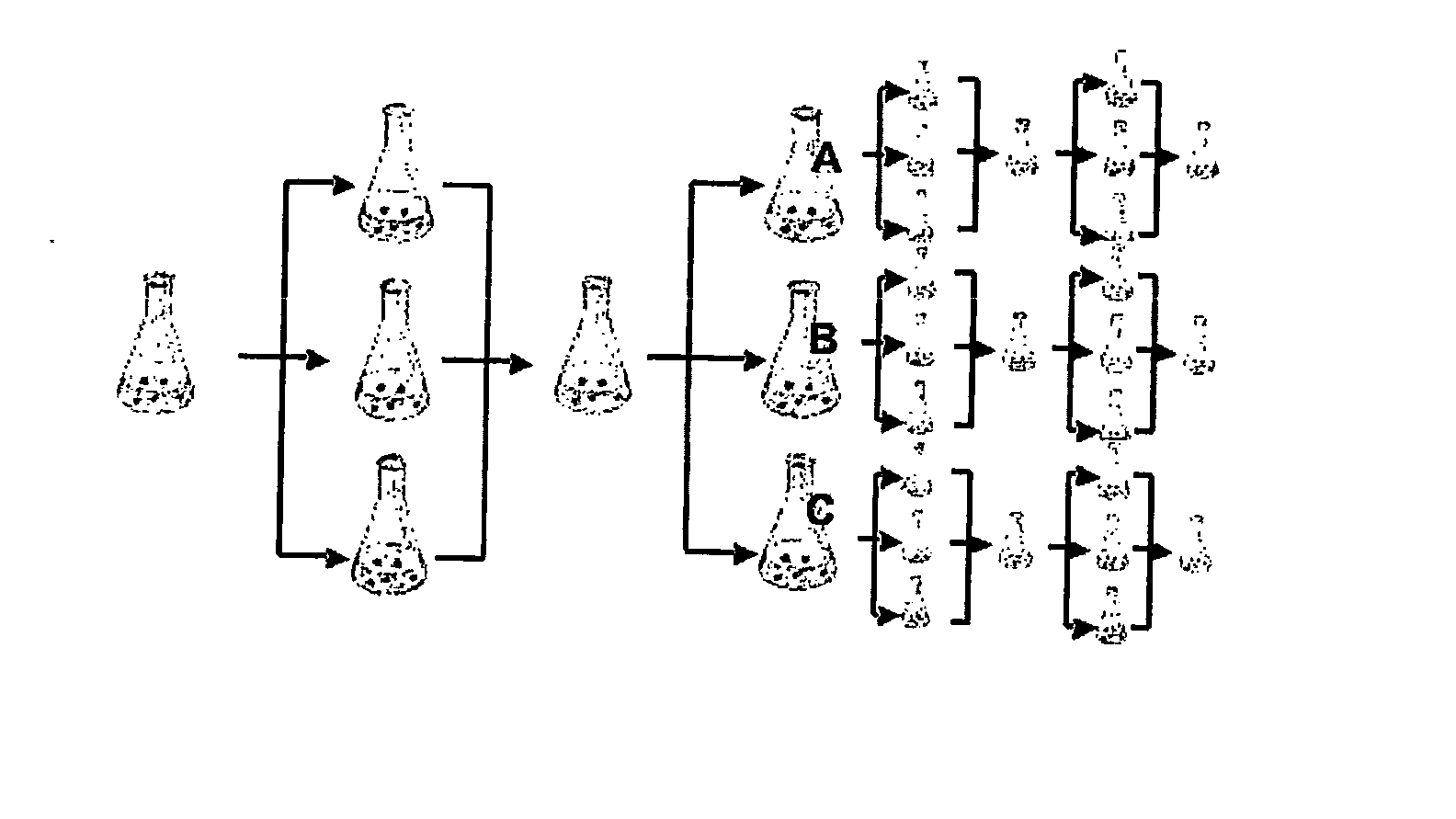
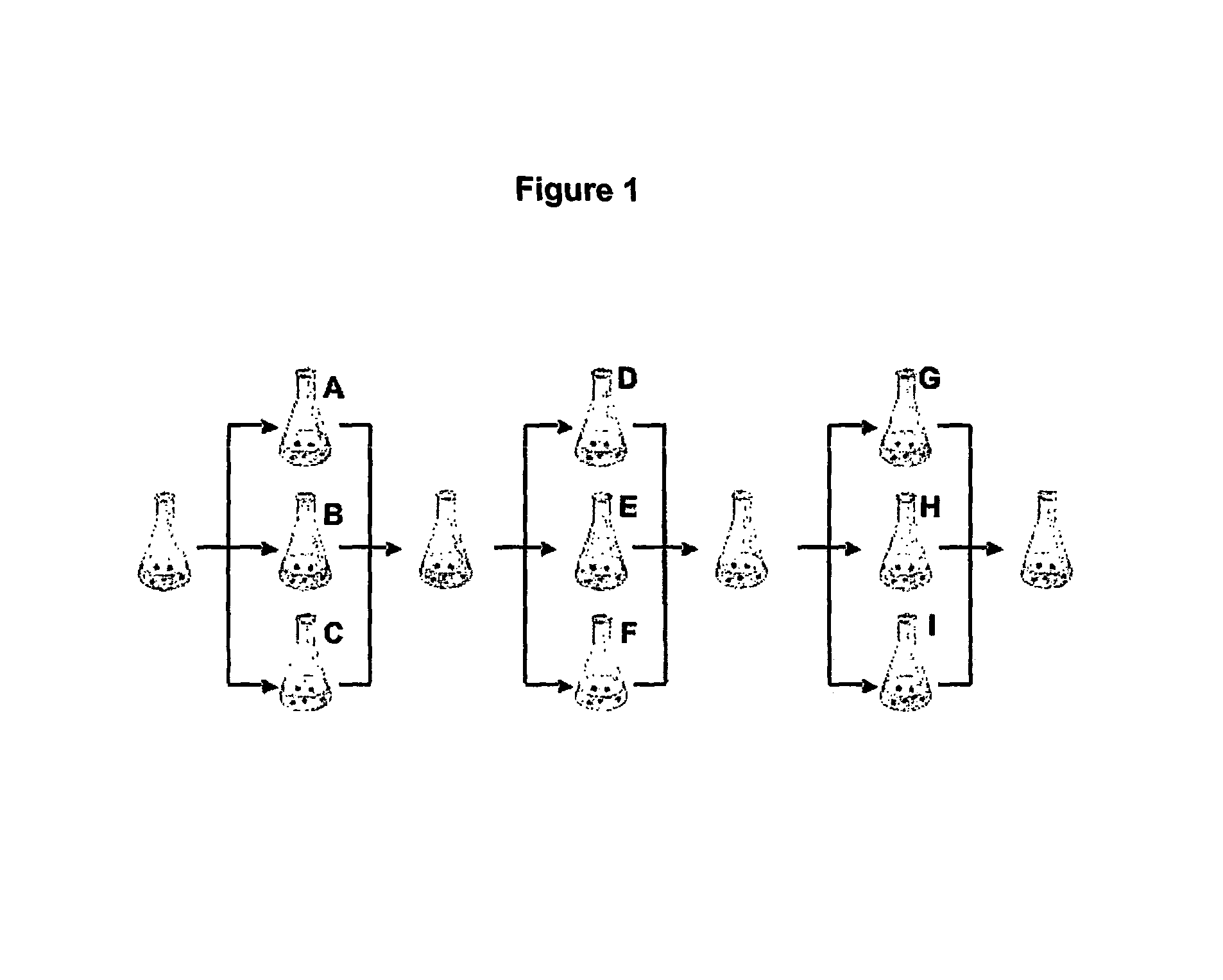
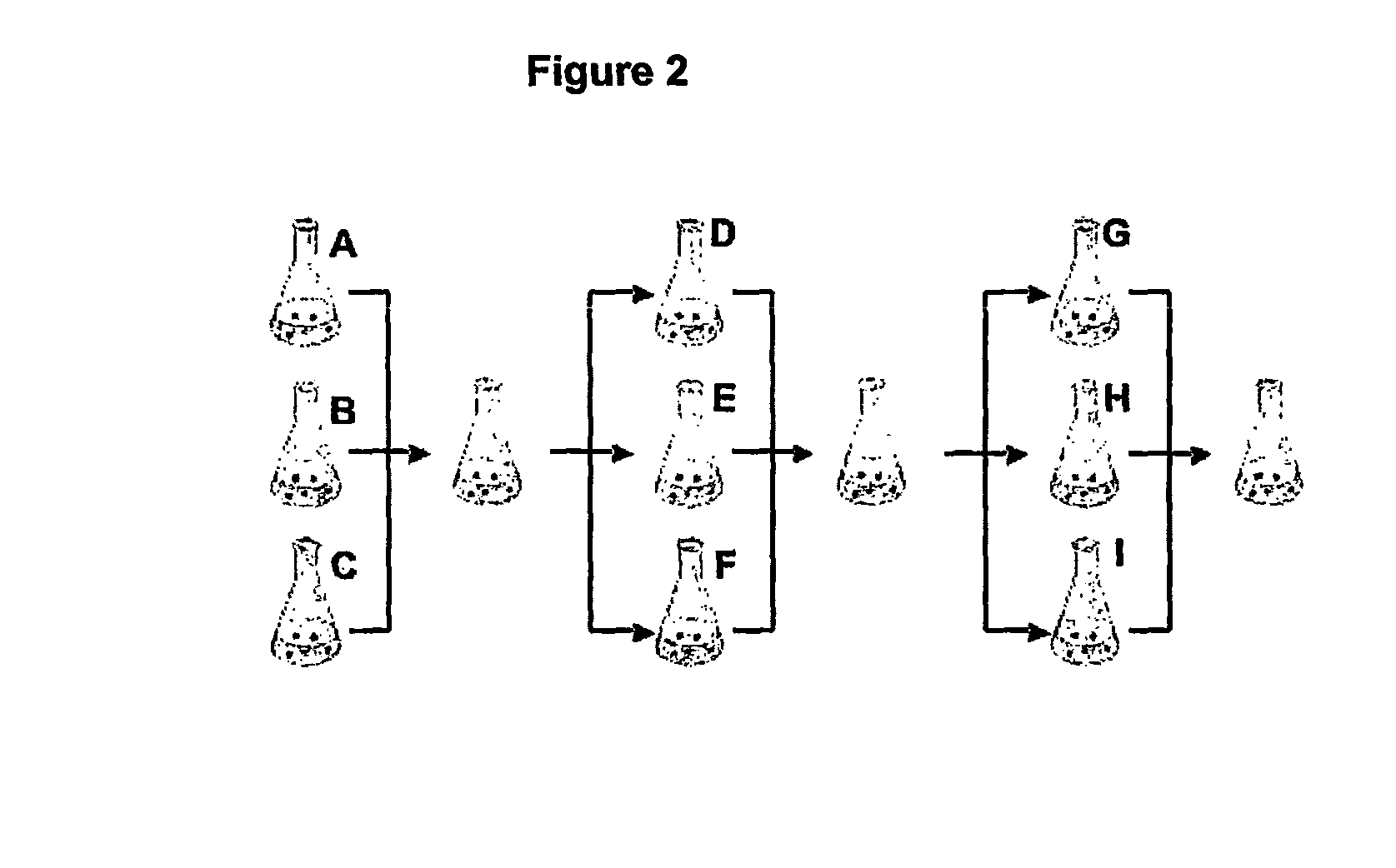
Cell Culture
a cell culture and cell technology, applied in the field of cell culture, can solve the problems of multi-stage procedures, inability to achieve empirical determination of tissue culture conditions in practice, and inability to achieve multi-stage procedures, etc., and achieve the effect of level of control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Differentiation of ES Cell Units Using Split-Pool Cell Culture
[0191] A split-pool culture experiment was performed in order to assay tissue culture conditions that might give rise to neurons of a dopaminergic phenotype using a starter culture of undifferentiated mouse ES cells.
[0192] Undifferentiated ES cells were grown on gelatin-coated tissue culture plates in the presence of 1,400 U ml −1 of leukemia inhibitory factor (LIF;Chemicon) in ES cell medium consisting of knockout Dulbecco's minimal essential medium (DMEM; GIBCO / BRL) supplemented with 15% FCS, 100 mM MEM nonessential amino acids, 0.55 mM 2-mercaptoethanol, L-glutamine, and antibiotics (all from GIBCO / BRL). To induce embryoid body (EB) formation the cells were dissociated into a single-cell suspension by 0.05% trypsin and 0.04% EDTA in PBS and plated onto nonadherent bacterial culture dishes at a density of 2-2.5×104 cells cm−2 in the medium described above. The EBs were formed for four days and then plated onto adhesiv...
example 2
Split-Pool Cell Culture of HepG2 Cell Units
[0205] A split-pool culture experiment was performed in order to assay tissue culture conditions that might affect a particular cellular process, namely the expression and / or activity of cytochrome P450 (CyP450) metabolic enzymes. Members of this class of enzyme, such as 1A1 and 1A2, can be assayed with the use of a substrate, ethoxyresorufin, that is enzymatically hydrolyzed to produce a product, resorufin, that has distinct fluorescence characteristics that can be used to measure CyP450 enzyme activity. The expression of CyP450 enzymes can be regulated by inducer molecules, such as β-naphthoflavone, and inhibitors such as α-naphthoflavone, quinidine, or aminotriazole. It is also possible for some molecules to induce expression of a CyP450 gene(s), but inhibit activity of the enzyme product of that gene. Therefore, complicated patterns of expression and activity can arise according to the pattern of serial exposure of cells to regulatory ...
example 3
Growth of Cell Units Comprising Pluripotent Stem Cells
[0208] Pluripotent mouse ES cells expressing a Tau-GFP fusion protein were maintained on a feeder layer of mitomycin C-treated SNL cells (a STO cell derivative) in ES cell medium consisting of Iscove's medium supplemented with 15% FCS, 0.55 mM 2-mercaptoethanol, L-glutamine, antibiotics (all from GIBCO / BRL), and 1,400 U ml −1 of leukemia inhibitory factor (Chemicon) and split 1:5 every other day. ES cells and feeder cells used for the formation of cell units were transferred to a gelatin-coated flask and cultured for one day in ES medium to reduce the number of feeder cells in the culture. ES cells were trypsinised from the gelatin plates and washed with FCS-containing medium, then incubated with either Cytopore 2 or Cytodex 3 microcarriers (Amersham Biosciences) that had been hydrated in PBS, sterilised by autoclaving or 70% ethanol treatment, then washed with ES medium. The ES cells were seeded at a density of about 10 cells / m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dry weight | aaaaa | aaaaa |
| size parameters | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com