Vascularized tissue graft
a vascularized tissue and graft technology, applied in the field of tissue engineering, can solve the problems of limited technique, limited vascularized tissue production, and inability to produce other vascularized tissues suitable for grafting, and achieve the effects of facilitating delivery, repairing tissue deficit, and augmenting inappropriately depleted tissu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Support Matrix
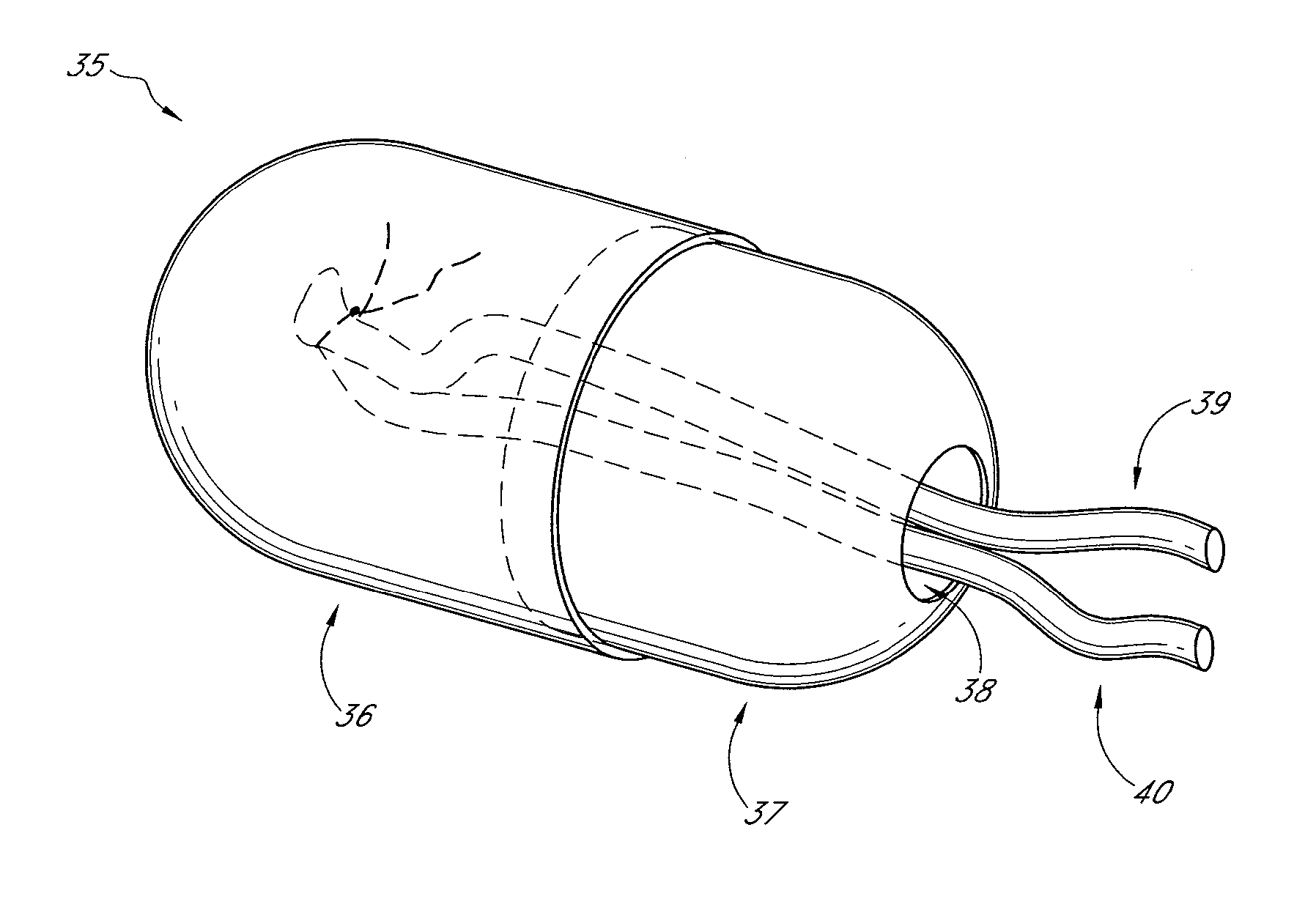
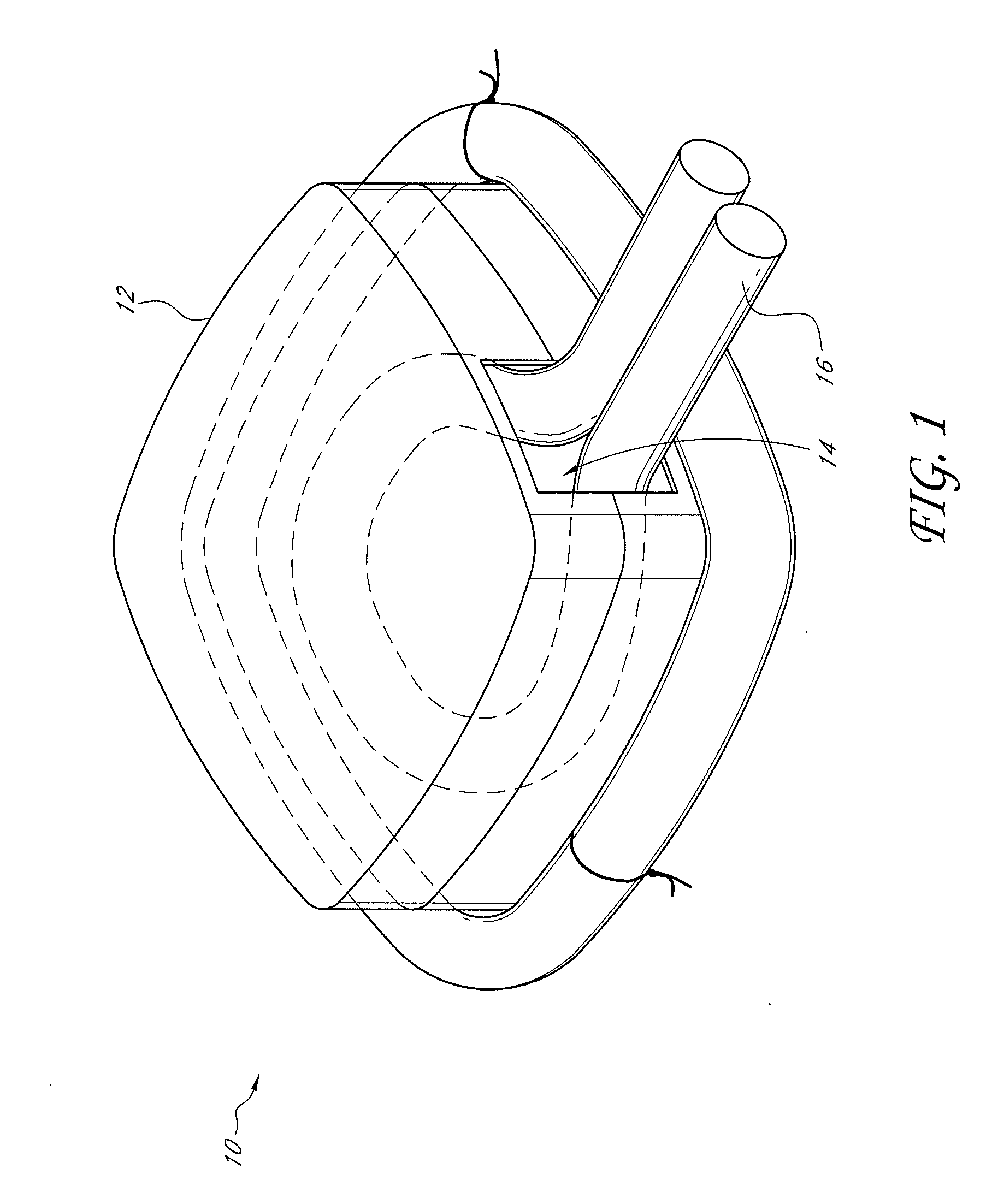
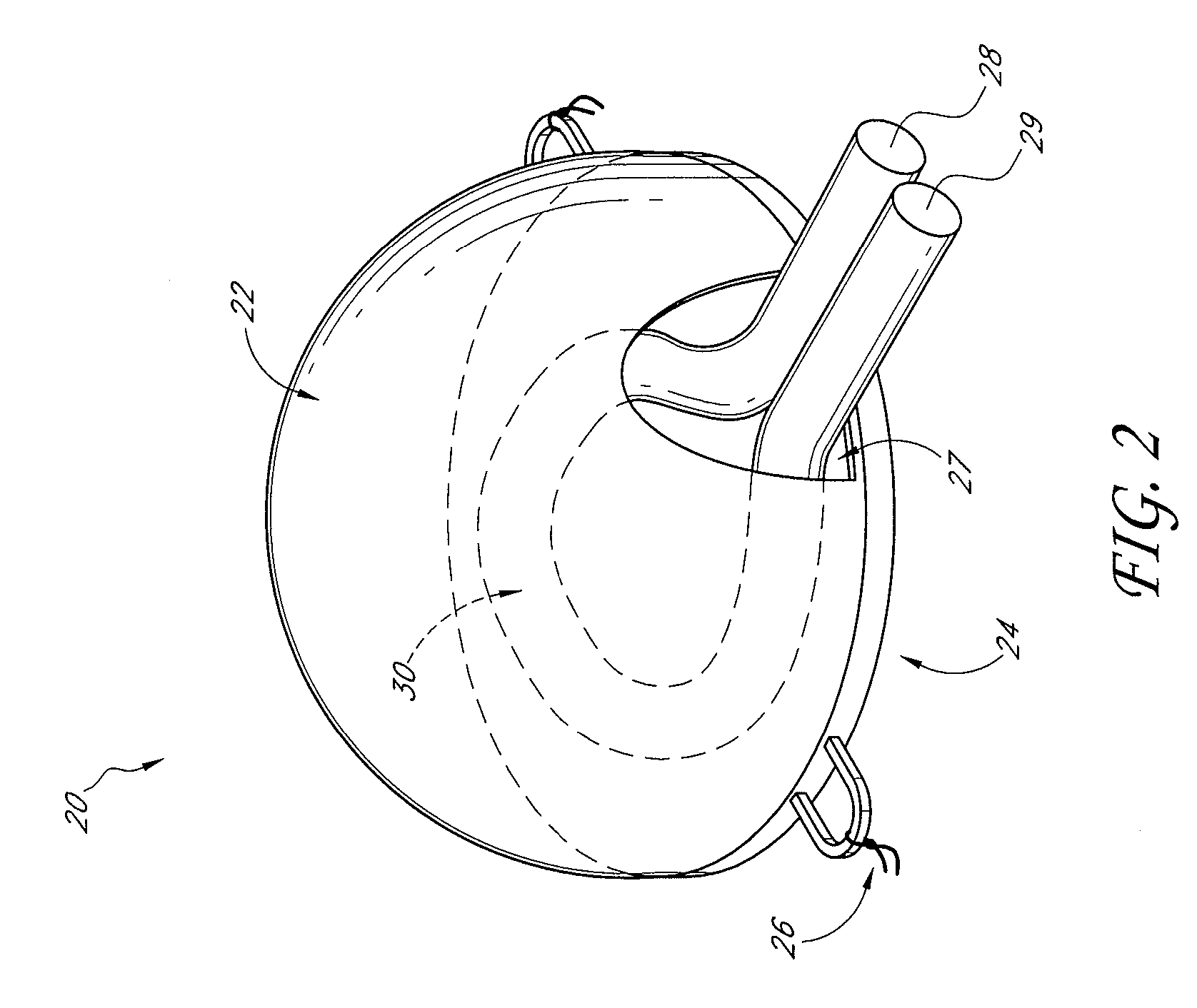
[0102] A custom-made polycarbonate chamber is prepared. It comprises a top and a bottom, and when the two halves are sealed together the internal volume is 0.45-0.50 mL. The general construction of the chamber 10 is illustrated in FIG. 1. In this exemplary chamber, the chamber is a transparent plastic cylindrical chamber with lid 12.
[0103] The basic chamber for use in rats is made of polycarbonate. In one variant, the chamber is made of polylactic acid or PLGA. The chamber is in the shape of a cylinder of external dimensions 14 mm diameter and 4 mm high, with a saw cut on one side to create an opening for the blood vessel entry and exit. Another variant has cut openings on opposite sides of the chamber to allow blood vessels to flow in one side and out the other. The chamber has a base and a removable lid 12. The base has holes to allow anchoring of the chamber to subcutaneous tissue. The internal volume is approximately 0.45-0.50 mL. The internal volu...
example 2
Creation of an AV Shunt Loop Inside the Tissue Chamber
[0114] The basic model has been described by Tanaka et al., 1996, supra. Briefly, male Sprague-Dawley rats (225-285 g) are anaesthetized with intraperitoneal phenobarbitone (50 mg / kg; 2.5 mL of a 6 mg / mL solution). Under sterile conditions an inferior-based flap is created in the right groin to expose the femoral vessels from the inguinal ligament to the superficial epigastric branch. A longitudinal incision was made in the left groin to harvest the left femoral vein from inguinal ligament to the superficial epigastric branch. This vein graft (approximately 1.5-3 cm long; usually 2 cm) was interposed between the recipient right femoral vein and artery at the level of the superficial epigastric artery by microsurgical techniques using 10-0 sutures. The shunt is placed into the chamber, the lid closed and the construct sutured to the groin musculature with the aid of small holes on the base of the chamber. An adipose layer was pla...
example 3
Assessment of Vascularization and Tissue Creation
[0116] At the specified time of exploration, the chamber is opened and the vessels cleaned and tested for patency. The vessels are tied off with a 5-0 silk suture at the entrance of the chamber and the flap harvested. In two of the five rats in each group, the flap is perfused via the aorta with India ink prior to harvest (details below). The flaps are assessed for volume and weight and placed in buffered 10% v / v formal saline (BFS) for histological examination. The animals are sacrificed with an intracardiac dose of sodium pentabarbitone (˜3 ml of 250 mg / m: solution) at the completion of the exploration.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| internal volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com