Polynucleotide Fragments of an Infectious Human Endogenous Retrovirus
a technology polynucleotide fragment, which is applied in the field can solve the problems that the infection of human endogenous retrovirus cannot be isolated or detected so far, and achieve the effect of improving antisense agent and greater resistance to enzymatic degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
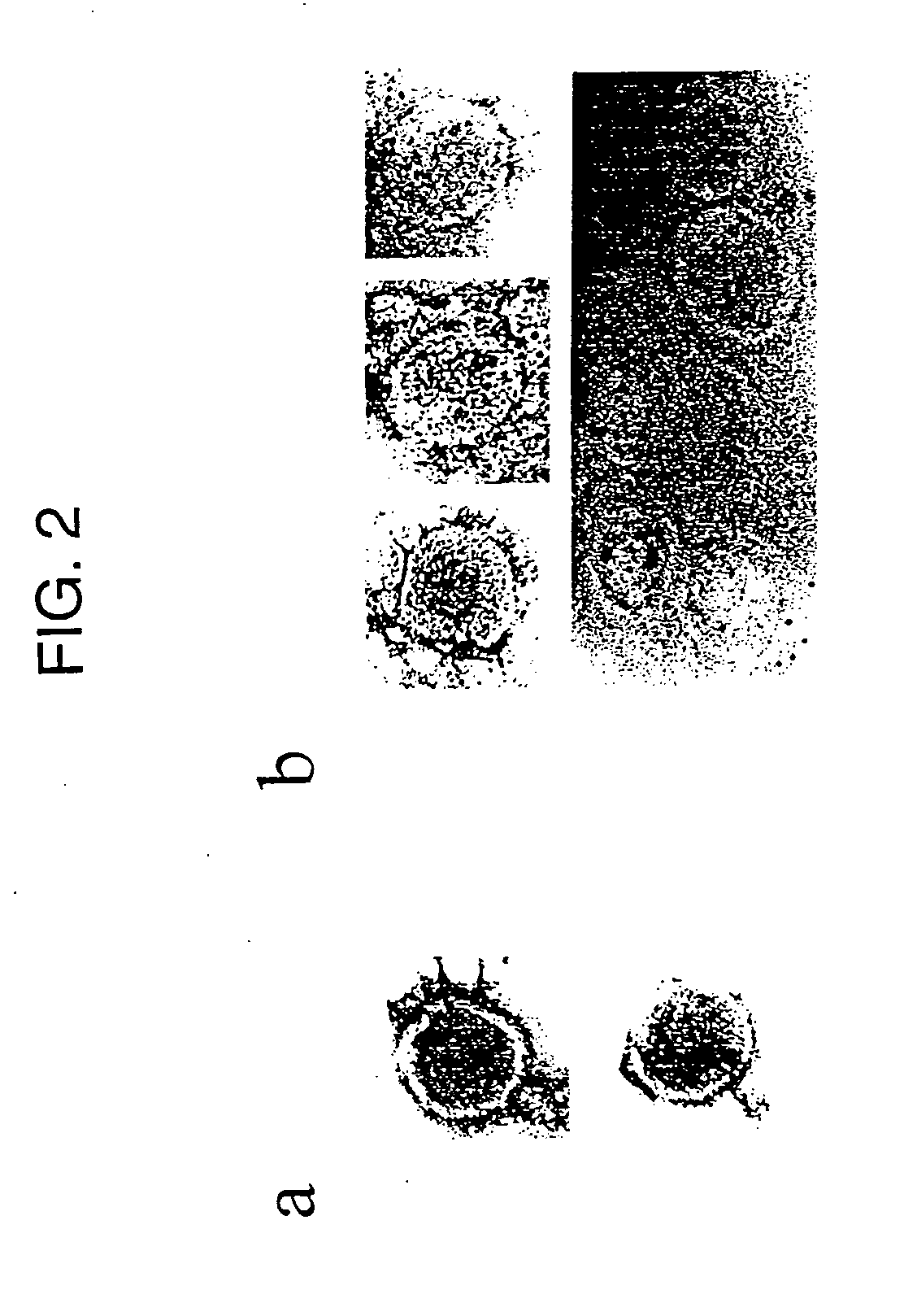
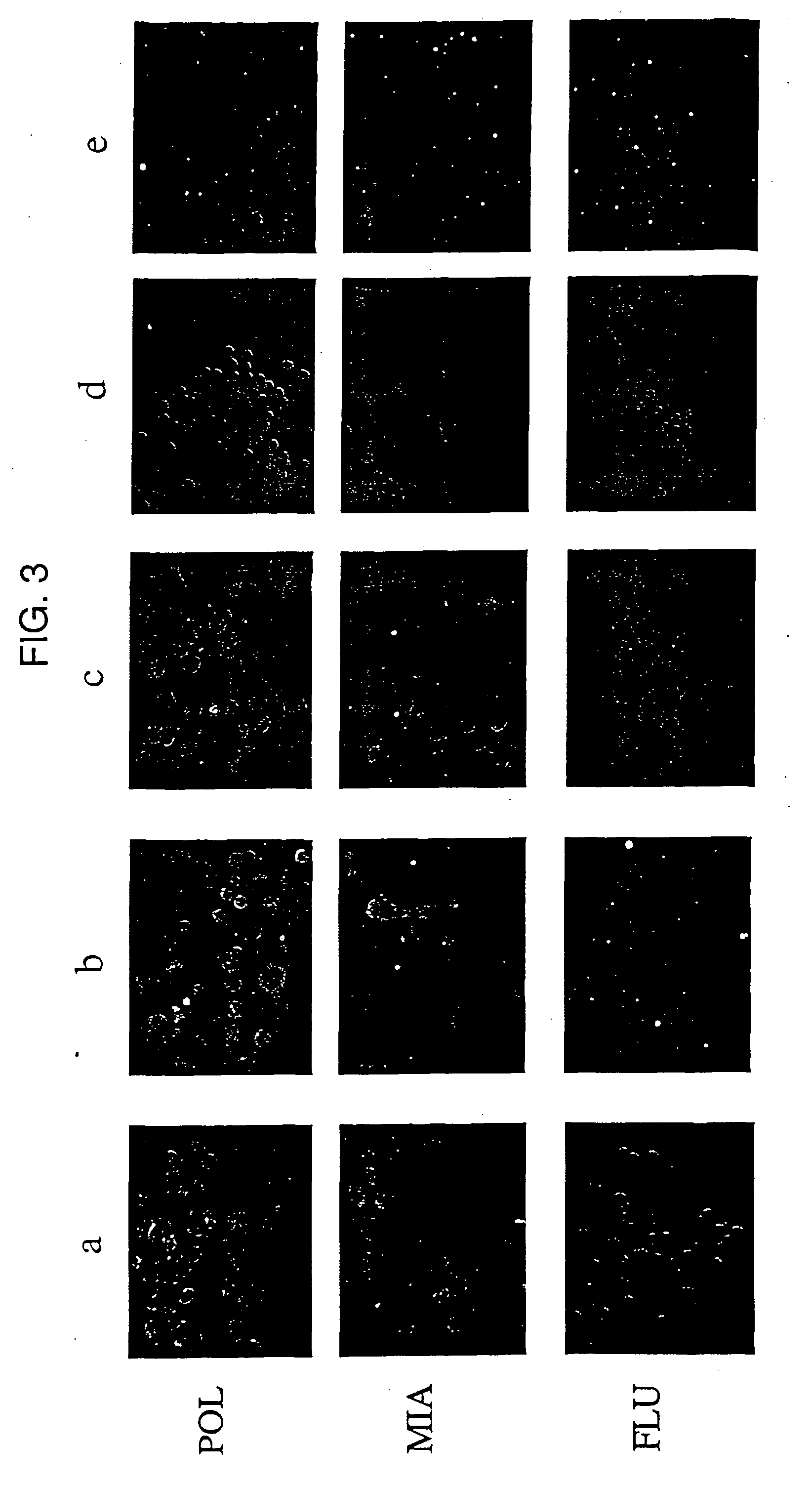
Human Melanoma Cells Contain RT Activity in their Supernatants
[0157] To detect extracellular virions, supernatants from the melanoma cell lines SKMel-28, SKMel-1, 518A2 were analyzed, as well as primary melanoma cells for pelletable RT. Supernatants from cultured human neonatal melanocytes (NHEM) served as controls. Cell-free supernatants from approximately 106 cells were concentrated by centrifugation. The RT activity of the resulting pellets was analyzed by F-PERT assay.
[0158] Supernatants from melanoma cell lines, and normal human melanocytes were centrifuged at 3000×g at 4° C. and sterile-filtered through a 0.22 μm low-protein membrane (Nunc) to remove cells and cellular debris. The clarified supernatants were centrifuged for 20 min at 250000×g at 4° C. in a Beckman SW50.1 rotor. Pellets were rinsed with phosphate-buffered saline (PBS) and centrifuged for 15 min at 250000×g and resuspended. The resulting particle suspensions were used as an enzyme source for RT assays.
[0159] ...
example 2
Human Melanoma Cell Derived Particles Package Sequences with High Homology to HERV-K
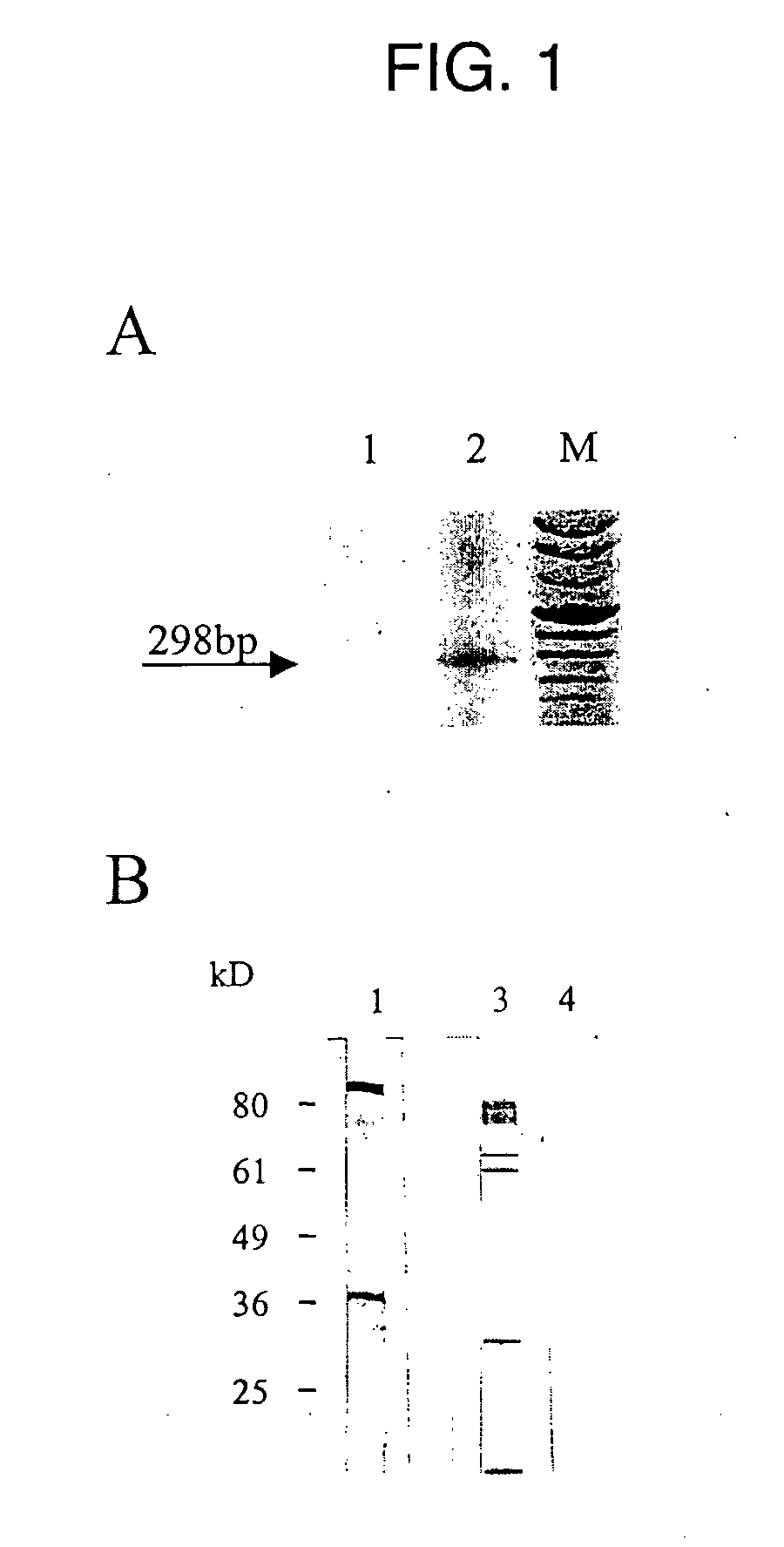
[0161] Retroviral pol genes are generally the most conserved sequences among retroviruses. In an attempt to analyze the particle preparations on a molecular level, a sequence was amplified by using degenerate primers corresponding to conserved sequences of the pol gene. Particle preparations of the melanoma cell line 518A2 and SK-Mel28 were used for RT-PCR analysis. Particles were treated with DNase, to remove contaminating traces of genomic DNA.
[0162] Oligonucleotide primers 3′ABDPOLS 5′dCATTCCTTGTGGTAAAACTTTCCA[T / C]TG 3′ (SEQ ID NO:57) and 5′ABDPOLAS 5′dCCCCTTGGAATACTCCTGTTTT[T / C]TG 3′ (SEQ ID NO:58) (Codon Genetic Systems, Austria), derived from conserved regions within the retroviral reverse transcriptase (RT), were adopted from Medstrand and Blomberg. In order to release viral RNA, the particle suspension was transferred to a 0.5 ml polypropylene microcentrifuge tube (Sorensen Inc., USA). Then...
example 3
Human Melanoma Derived Particles Contain Mature gag and env Proteins
[0165] Particles derived from SK-Mel28 melanoma cells were purified on iodixanol-cushions and analyzed for the presence of HERV-K-specific env and gag proteins in Western blots.
[0166] Iodixanol-cushion purified particles from SK-Mel28 supernatants were pelleted, resuspended in PBS and analyzed. The amount of soluble proteins was quantified by means of a modified Bradford analysis (Bio-rad, Richmond Calif.). 5 μg of total protein was applied per lane and separated by SDS-PAGE (10%). Proteins were transferred to PVDF membranes (Millipore, Bedford, Mass.) by Western blotting, and generated blots were incubated with the gag- and env-specific antisera. The membranes were washed twice with blocking solution and HERV-K-specific proteins were detected with an alkaline-phosphatase-conjugated second-step antibody.
[0167] The envelope (env) gene of retroviruses displays an ORF for the surface protein (SU) and a membrane span...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com