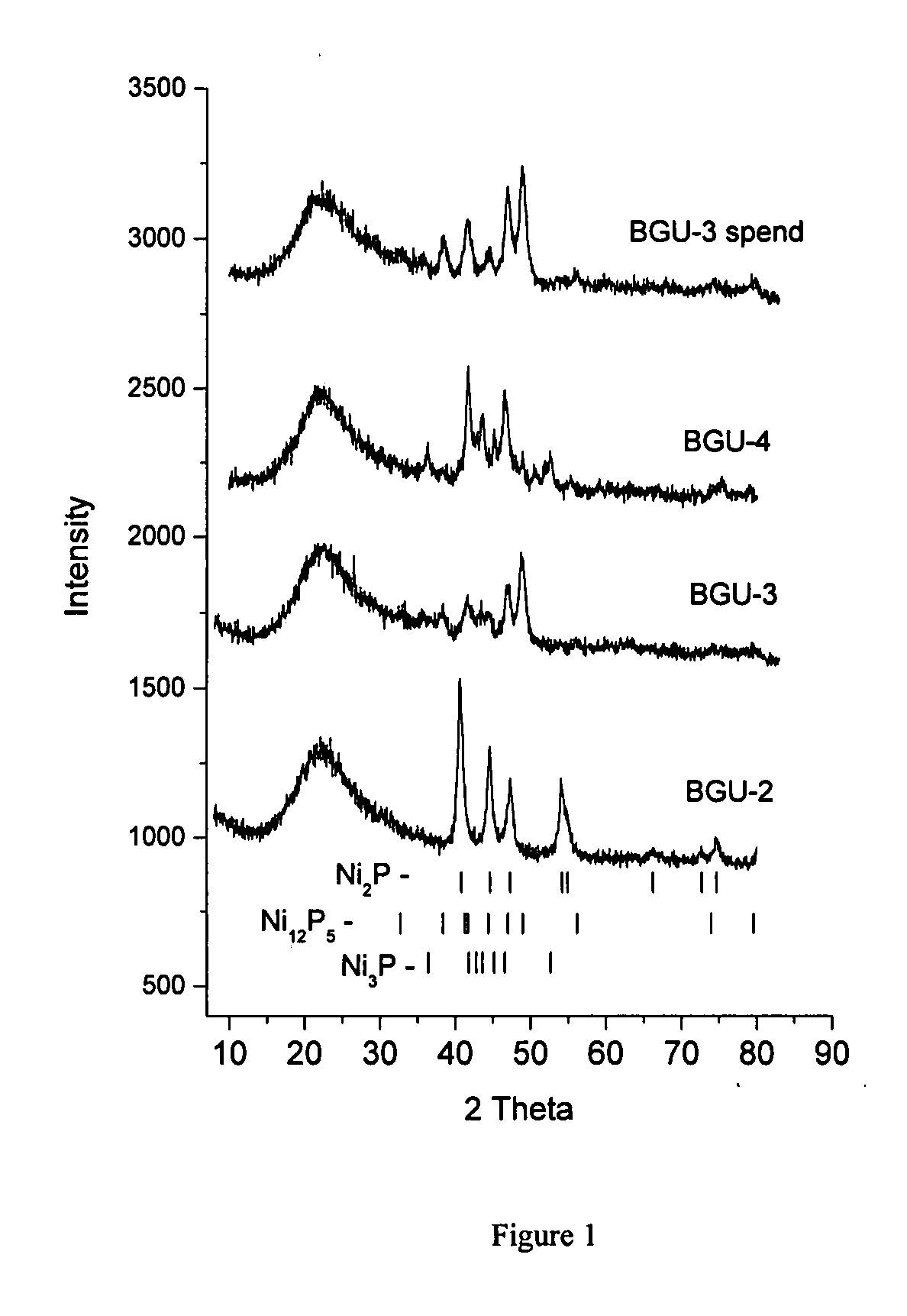
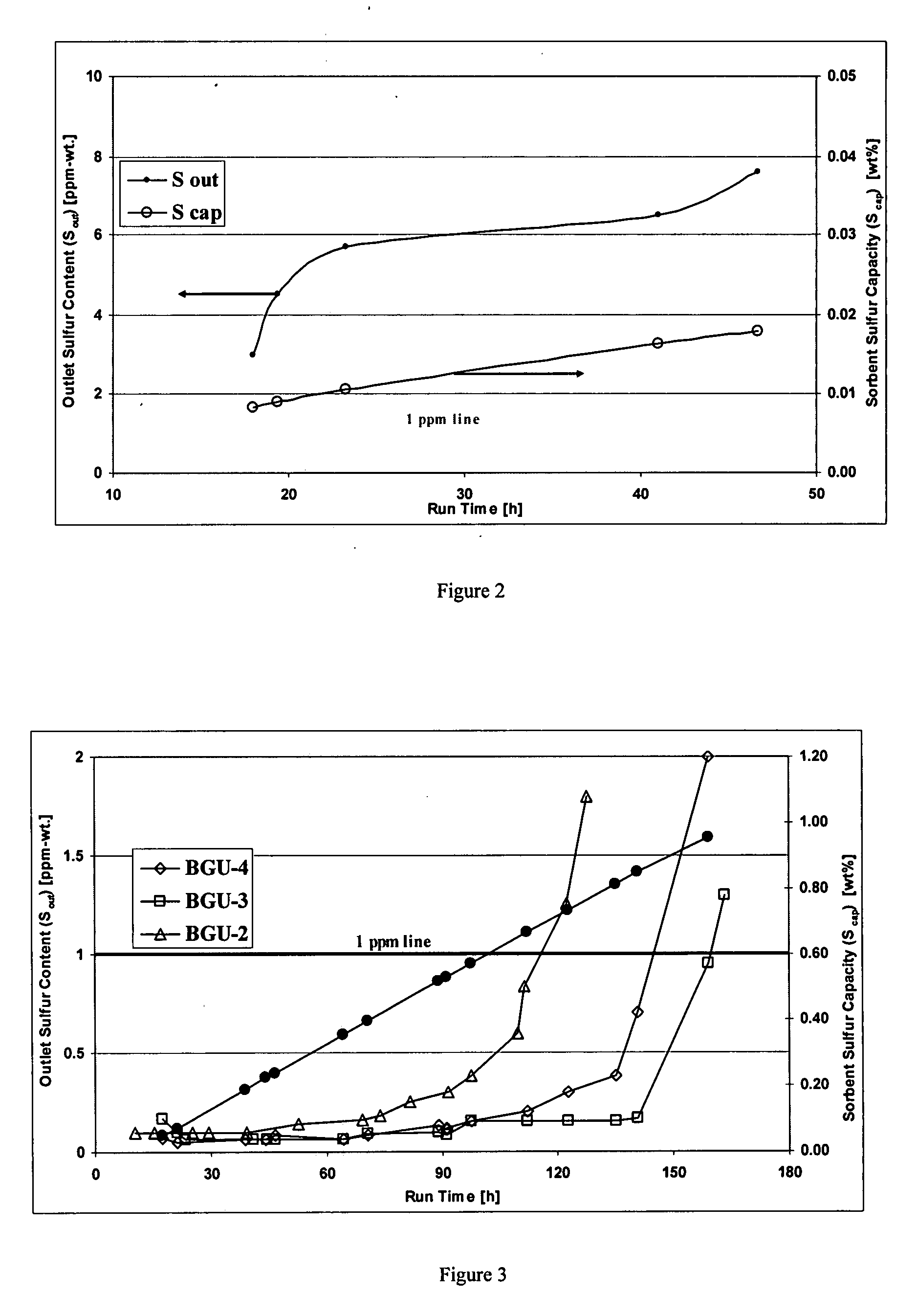
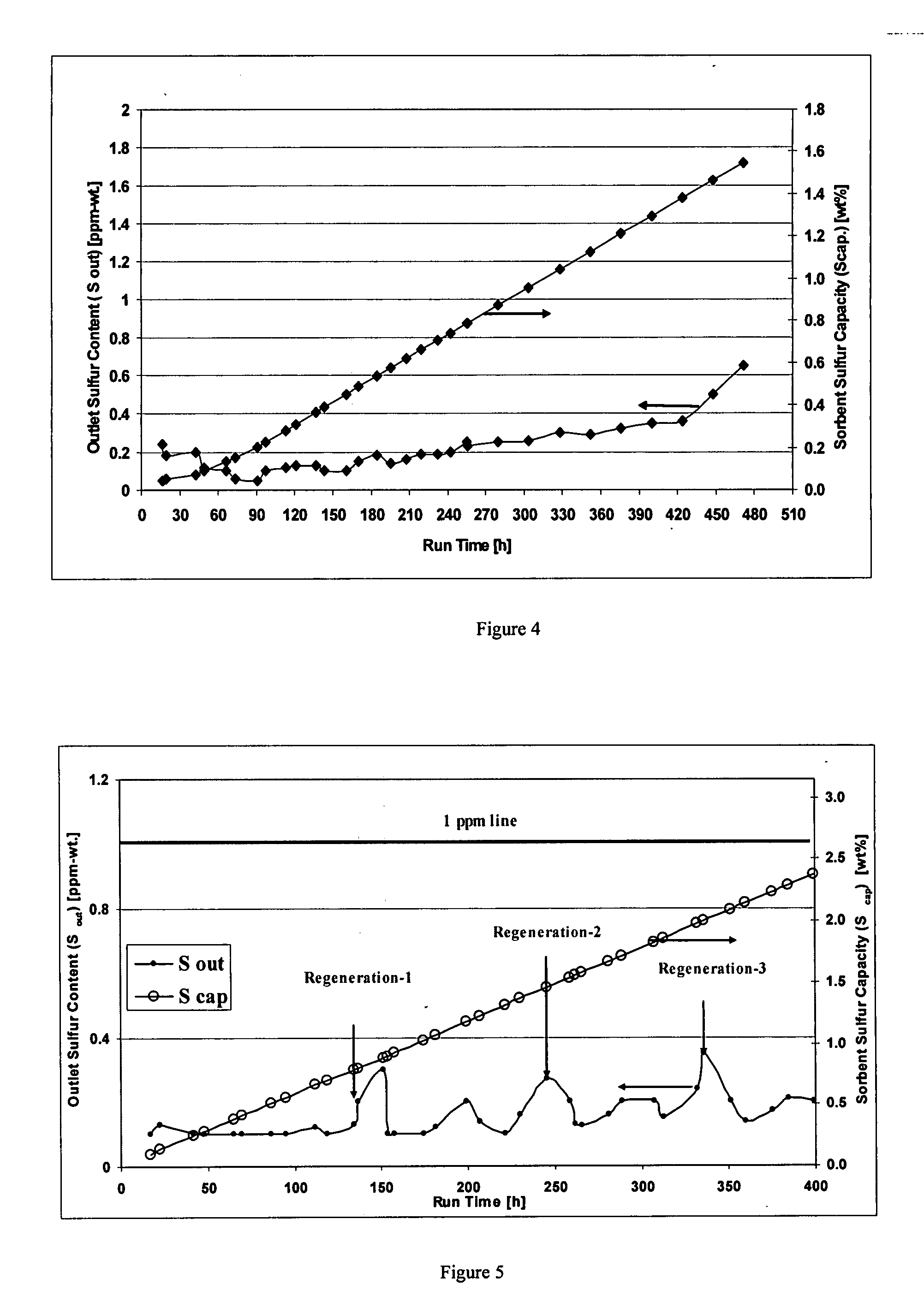
Process for adsorption of sulfur compounds from hydrocarbon streams
a technology of hydrocarbons and sulfur compounds, applied in the field of process for adsorption of sulfur compounds from hydrocarbon streams, can solve the problems of low capacity and sorbent non-regenerability, affecting the commercial application of such process for ultradeep desulfurization of diesel fuel, and limiting the sulfur capacity of ni° phase even at high nickel dispersion of >30%, etc., to achieve the effect of increasing the loading of the nickel phosphide complex
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Comparative
[0024] In 250 ml flask placed in a heating bath, provided by magnetic stirrer and condenser, 5 g of silica gel (PROMEKS, PI-258) calcined at 500° C. for 2 h with surface area of 220 m2 / g and pore diameter of 26 nm was placed with a mixture of two solutions 0.5 g aluminum tri-sec butoxide with 100 mL toluene, and 1.5 g triethylamine with 100 mL. The toluene suspension was vigorously stirred at 85° C. for 6 h, and then the solid was separated by filtration. The alumina-grafted mesoporous silica solid was suspended in 150 mL of ethanol solution containing 0.22 g of water and it was stirred at room temperature for 24 h. The alumina-grafted mesoporous silica solid then filtered and dried with vacuum at 85° C. for 2 h, followed by gradual calcinations in periods of 2 hours at temperatures 250° C. and 400° C. and then calcinated in air for 4 hours at 500° C. The alumina-grafted mesoporous silica material exhibit surface area of 243 m2 / g and a narrow mesopore size distribution, ...
example 2
[0028] A sample of 6 g of silica gel (PROMEKS, PI-258) with surface area of 220 m2 / g and pore diameter of 26 nm was calcined for 2 hours at 500° C. for 2 h. Its water capacity at the wetness point was 2.7 cc (H2O) / g. 16.2 ml of transparent solution was prepared by mixing 6 ml of distilled H2O and 2.5 ml of 68% HNO3, adding 9 g of Ni(NO3)2*6H2O (0.031 mol Ni) and slowly inserting 4.1 g of (NH4)2HPO4 (0.031 mol P). Stirring was continued for 30 min until all the salts were dissolved yielding a transparent, green solution with pH of 4.0. The solution was inserted inside the pores of silica gel by incipient wetness method. The impregnated material was dried for 4 hours in air at 120° C. (heating rate 5° C. / min) and then calcined for 6 hours at 500° C. (heating rate 1° C. / min). EDX analysis of the calcined composite indicated the content of Ni, P, Si, O to be 22.9, 10.9, 35.1 and 31.1 wt % respectively and the atomic ratio of Ni / P was 1.1.
[0029] 0.5 g of the above composite material was...
example 3
[0030] A sample of 10 g of silica gel (PQ Co-PM5308) with surface area of 480 m2 / g and average pore diameter of 10 nm was calcined for 2 hours at 500° C. Its water capacity at the wetness point was 2.31 cc (H2O) / g (silica). 23 ml of transparent solution was prepared by mixing 8 ml of distilled H2O and 3.25 ml of 68% HNO3, adding 16.25 g of Ni(NO3)2*6H2O (0.056 mol Ni) and slowly inserting 3.75 g of (NH4)2HPO4 (0.028 mol P). Stirring was continued for 30 min until all the salts were dissolved yielding a transparent, green solution with pH of 3.5. The impregnated material was dried for 4 h in air at 120° C. (heating rate 5° C. / min) and then calcined for 6 h at 500° C. (heating rate 1° C. / min). EDX analysis of the calcined composite indicated the content of Ni, P, Si, O to be 26.2, 6.4, 38.9 and 28.5 wt % respectively and the atomic ratio of Ni / P was 1.97.
[0031] 0.5 g of the above composite material was reduced in a quartz reactor under atmospheric pressure with an H2 flux of 1000 cc*...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com