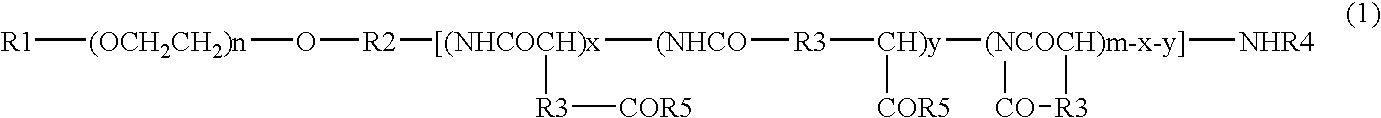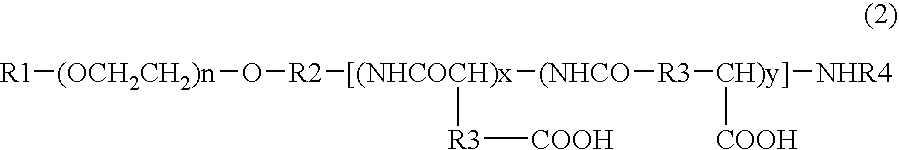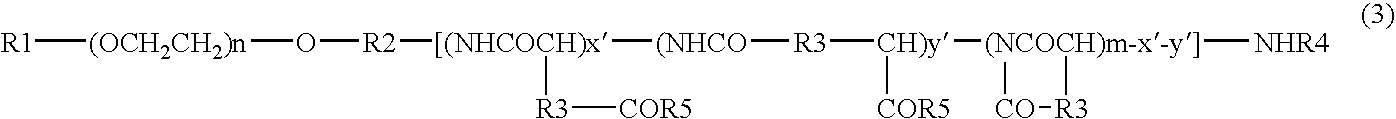Novel Block Copolymer, Micelle Preparation, And Anticancer Agent Containing The Same As Active Ingredient
a technology of micelle and anticancer agent, which is applied in the direction of biocide, drug composition, animal husbandry, etc., can solve the problems of reduced stability of pharmaceutical preparation and difficult administration, and achieve the effects of less toxicity, increased water solubility of drug, and more potent drug activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Block Copolymer 2
[0080]DMF (630 mL) was added to 42.00 g of PEG (average molecular weight 12000)-pAsp (polyaspartic acid; average polymerization degree 40)-Ac (represented by the general formula (2) wherein R1 is a methyl group, R2 is a trimethylene group, R3 is a methylene group, R4 is an acetyl group, n is about 272, x is about 10, y is about 30; abbreviated hereinafter as PEG-pAsp-Ac) produced by a method described in JP-A-6-206815 (Patent Document 2) supra, and PEG-pAsp-Ac was dissolved at 25° C., and DMAP (9.90 g), 4-phenyl-1-butanol (10.93 mL) and DIPCI (15.86 mL) were added thereto and reacted at the same temperature for 24 hours. 1.58 L of ethyl acetate and then 4.73 L of hexane were added to the reaction liquid, and precipitates were collected by filtration and dried under reduced pressure to give 49.56 g crude crystals. The crude crystals were dissolved in acetonitrile containing 50% water (hereinafter referred to as “50% hydrous acetonitrile”), then passed t...
example 2
Production of Block Copolymer 5
[0103]PEG-pAsp-Ac (3.0 g) produced by a method described in JP-A-6-206815 (Patent Document 2) was dissolved in DMF (120 mL), and benzyl bromide (0.60 mL) and 1,8-diazabicyclo[5.4.0]undec-7-ene (0.75 mL) were added thereto and reacted at 35° C. for 17 hours. This reaction liquid was added dropwise to a mixed solvent (1.2 L) consisting of diisopropyl ether:ethanol (4:1), and precipitates were recovered by filtration and dried under reduced pressure to give 3.17 g of crude crystals. The crude crystals were dissolved in 30% aqueous acetonitrile solution and then passed through cation-exchange resin Dowex 50w8 (40 mL) and washed with the 30% aqueous acetonitrile. The eluent was concentrated under reduced pressure and lyophilized to give 2.99 g of block copolymer 4.
[0104]The block copolymer 4 (19.5 mg) was hydrolyzed by the same method as in Example 1 and measured by reverse phase HPLC, indicating that benzyl alcohol bound via an ester linkage was 32% relati...
example 3
Production of Block Copolymer 7
[0111]DMF (30 mL) was added to PEG-pAsp-Ac (2.0 g) produced by a method described in JP-A-6-206815 (Patent Document 2), to dissolve it at 25° C., and DMAP (0.472 g), benzyl alcohol (499 μL) and DIPCI (755 μL) were added thereto and reacted at the same temperature for 21 hours. 75 mL of ethyl acetate and then 225 mL of hexane were added to the reaction liquid, and precipitates were collected by filtration and dried under reduced pressure to give 2.28 g of crude crystals. The crude crystals were dissolved in 50% hydrous acetonitrile, then passed through cation-exchange resin Dowex 50w8 (30 mL) and washed with 50% hydrous acetonitrile. The eluent was concentrated under reduced pressure and lyophilized to give 2.10 g of block copolymer 6.
[0112]The block copolymer 6 (35.5 mg) was hydrolyzed by the same method as in Example 1 and measured by reverse phase HPLC, indicating that benzyl alcohol bound via an ester linkage was 60% relative to m.
[0113]When the blo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com