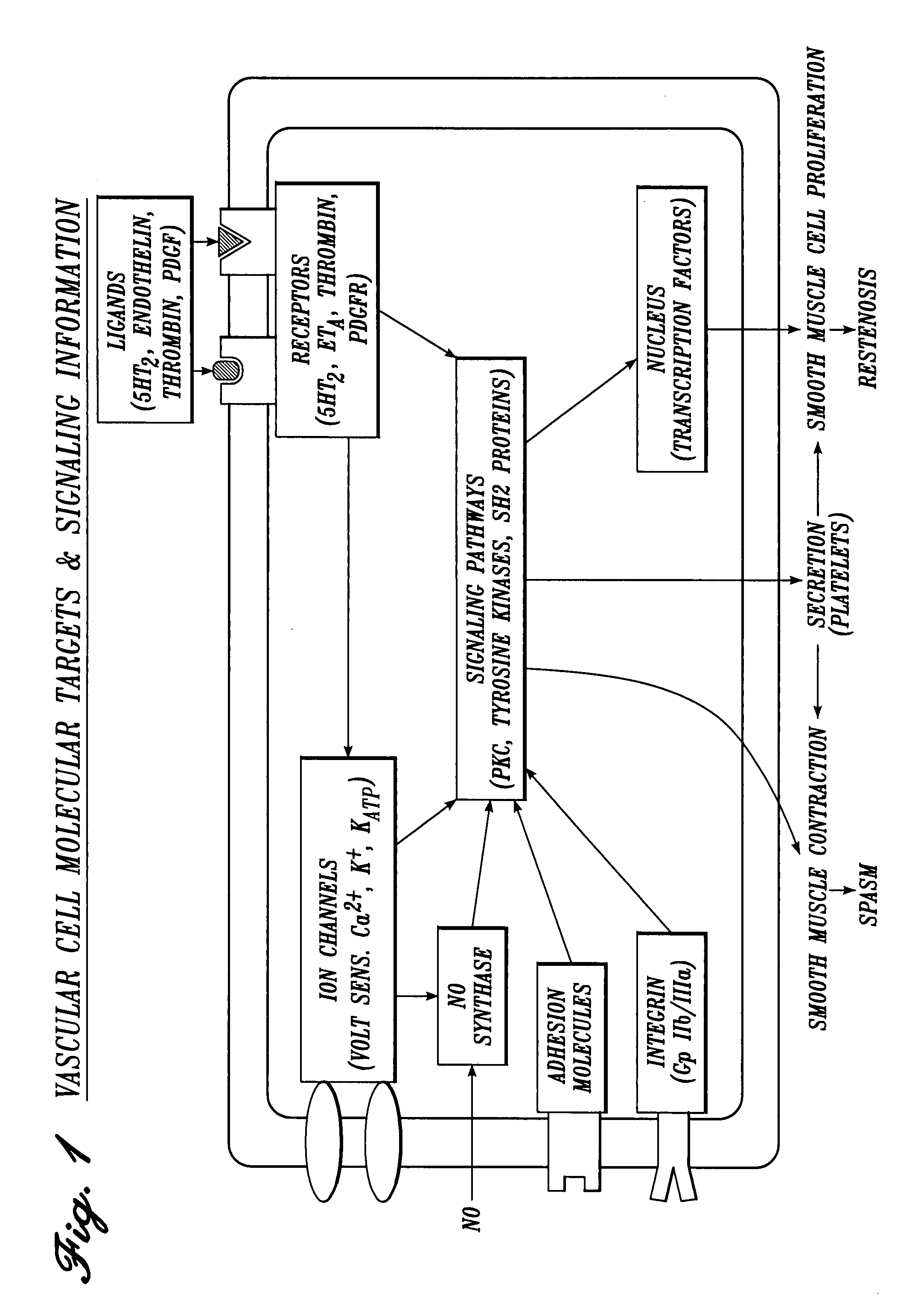
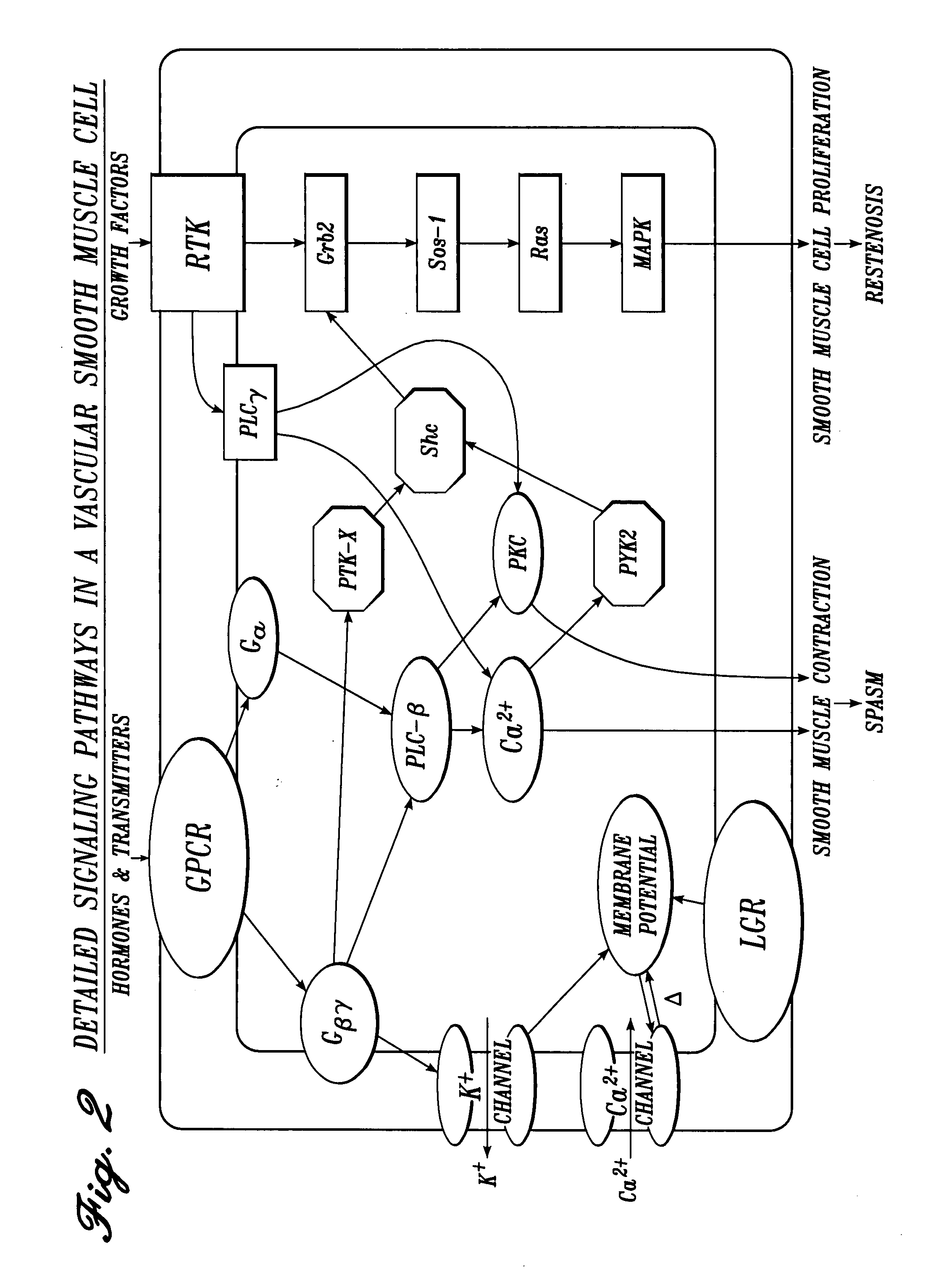
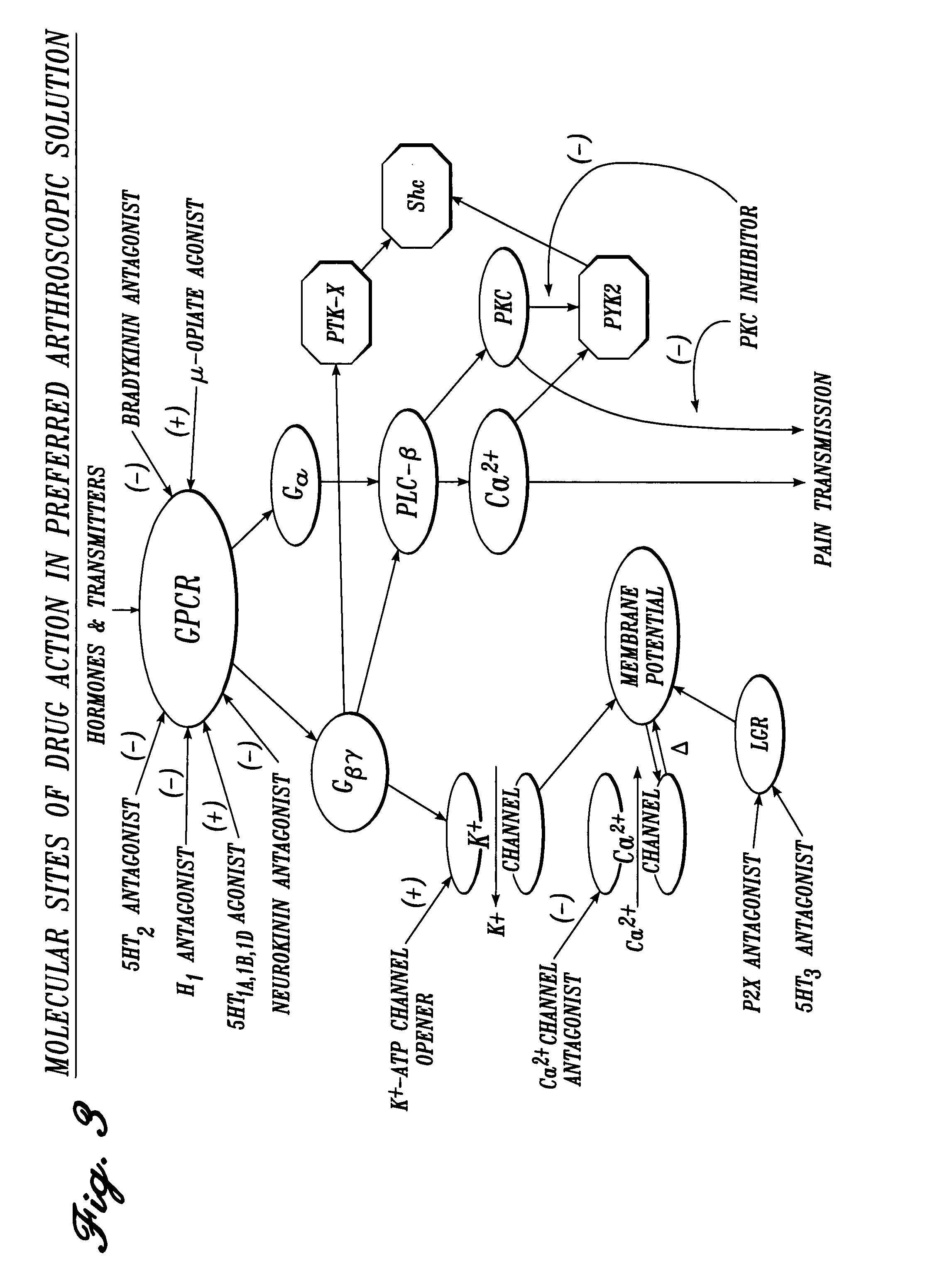
Cardiovascular compositions and methods using combinations of anti-platelet agents
a cardiovascular composition and anti-platelet technology, applied in the direction of drug compositions, cardiovascular disorders, cyclic peptide ingredients, etc., can solve the problems of high incidence of nausea and vomiting related to opioids, inability to develop therapeutic agents aimed at treating postoperative pain while avoiding detrimental side effects, and inability to provide analgesics and anti-inflammatory, anti-spasm and anti-restenotic effects of conventional physiologic irrigation fluids. to achieve the effect of decreasing th
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
A. Example I
Irrigation Solution for Arthroscopy
[0148]The following composition is suitable for use in anatomic joint irrigation during arthroscopic procedures. Each drug is solubilized in a carrier fluid containing physiologic electrolytes, such as normal saline or lactated Ringer's solution, as are the remaining solutions described in subsequent examples.
TABLE 25Concentration(Nanomolar):MostClass of AgentDrugTherapeuticPreferredPreferredserotonin2 antagonistamitriptyline0.1-1,000 50-500100serotonin3 antagonistmetoclopramide10-10,000 200-2,0001,000histamine1 antagonistamitriptyline0.1-1,000 50-500200serotonin1A, 1B, 1D, 1Fsumatriptan1-1,00010-20050agonistbradykinin1 antagonist[des-Arg10]1-1,00050-500200derivative ofHOE 140bradykinin2 antagonistHOE 1401-1,00050-500200
example ii
B. Example II
Irrigation Solution for Cardiovascular and General Vascular Therapeutic and Diagnostic Procedures
[0149]The following drugs and concentration ranges in solution in a physiologic carrier fluid are suitable for use in irrigating operative sites during cardiovascular and general vascular procedures.
TABLE 26Concentration(Nanomolar):MostClass of AgentDrugTherapeuticPreferredPreferredserotonin2 antagonisttrazodone0.1-2,00050-500200serotonin3 antagonistmetoclopramide 10-10,000 200-2,0001,000serotonin1B antagonistyohimbine0.1-1,00050-500200bradykinin1 antagonist[des-Arg10] 1-1,00050-500200derivative ofHOE 140cyclooxygenase inhibitorketorolac 100-10,000 500-5,0003,000
example iii
C. Example III
Irrigation Solution for Urologic Procedures
[0150]The following drugs and concentration ranges in solution in a physiologic carrier fluid are suitable for use in irrigating operative sites during urologic procedures.
TABLE 27Concentration(Nanomolar):MostClass of AgentDrugTherapeuticPreferredPreferredhistamine1 antagonistterfenadine0.1-1,000 50-500200serotonin3 antagonistmetoclopramide10-10,000 200-2,0001,000bradykinin1 antagonist[des-Arg10]1-1,00050-500200derivative of HOE140bradykinin2 antagonistHOE 1401-1,00050-500200cyclooxygenase inhibitor100-10,000 500-5,0003,000
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
| platelet aggregation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com