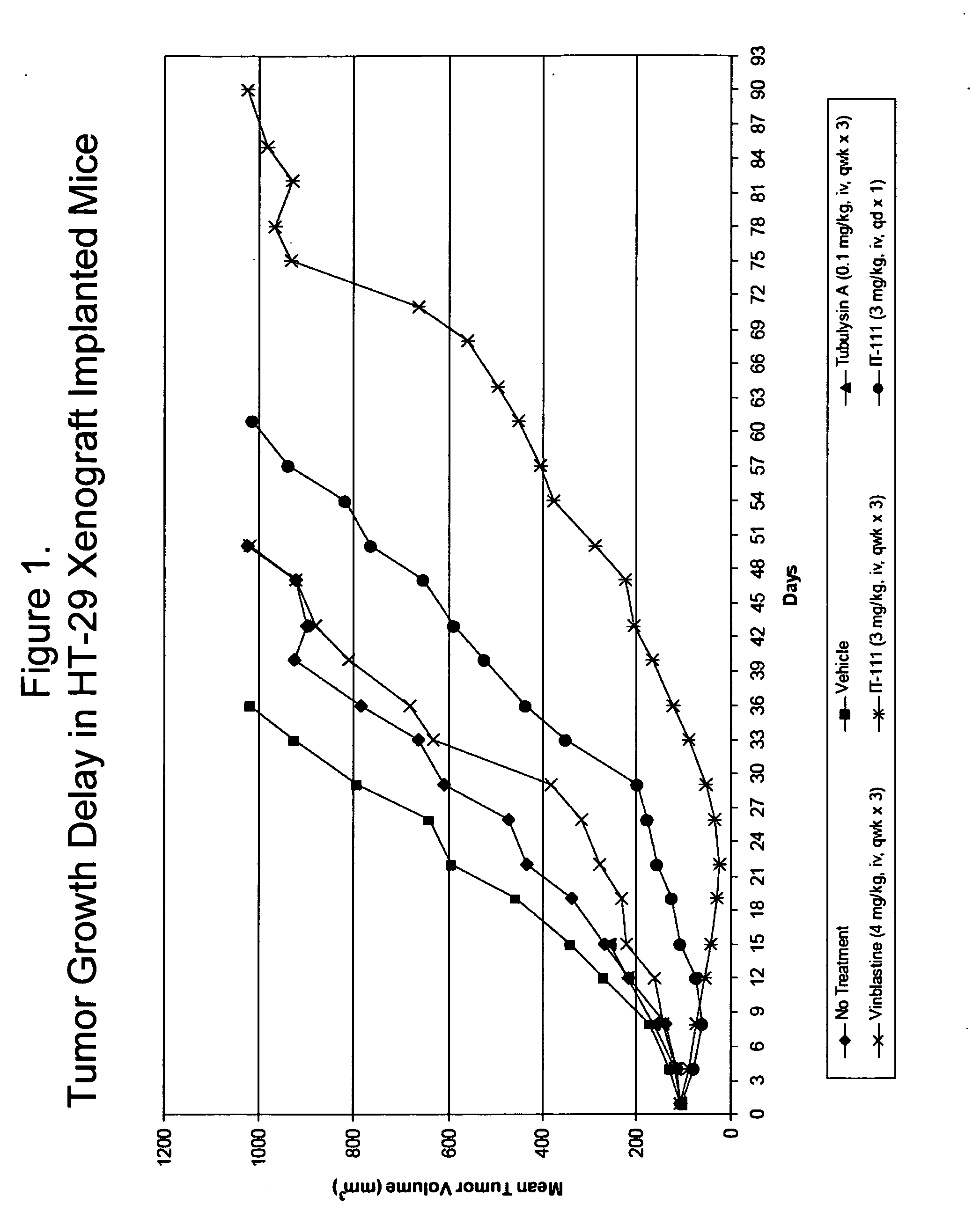
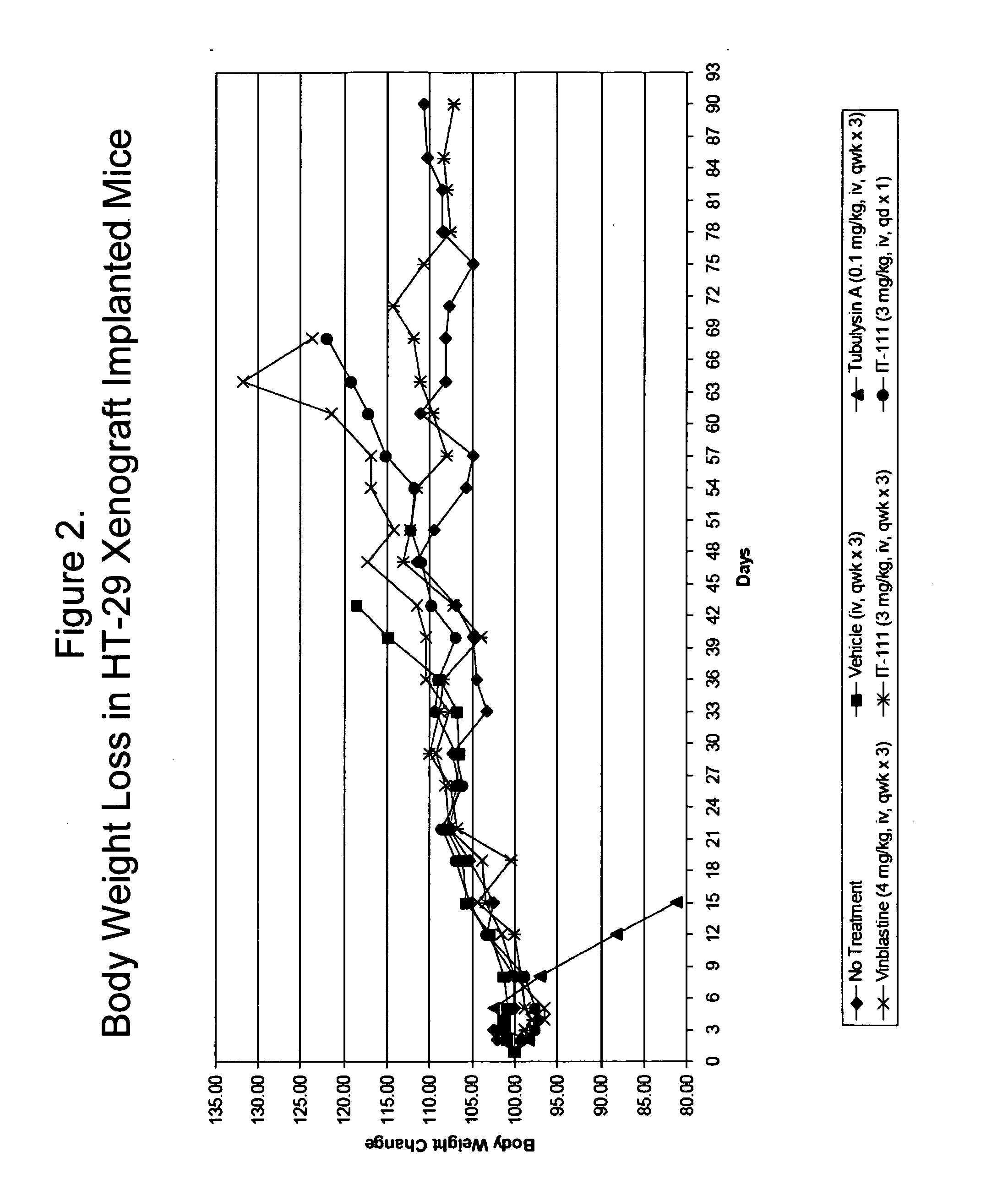
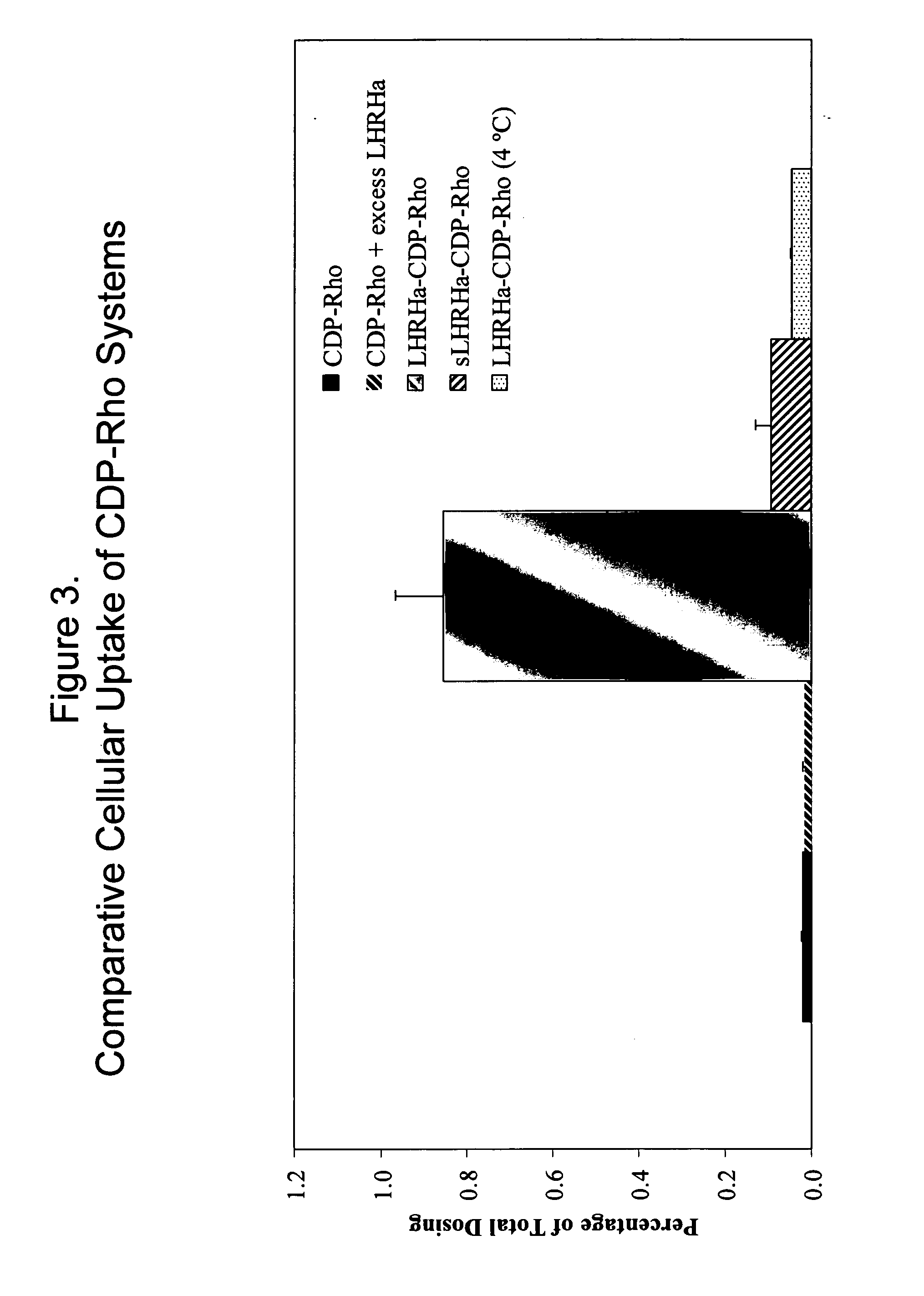
Cyclodextrin-based polymers for therapeutics delivery
a technology of cyclodextrin and polymer, applied in the direction of extracellular fluid disorder, immunological disorder, metabolism disorder, etc., can solve the problems of toxic side effects, pharmacological profiles, drug delivery of some small molecule therapeutic agents, etc., and achieve the effect of facilitating endocytosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of CDP-PEG-GFLG-MEDA-ETOP
[0611]
[0612]Fmoc-PEG-aceticacid (5.7 g, 13 mmol), HBTU (4.9 g, 13 mmol), HOBT (2.0 g, 13 mmol), and DIPEA (3.4 g, 26 mmol) were dissolved in DMF (25 mL). GFLG-MEDA-Z (5.1 g, 8.8 mmol) was dissolved in DMF (13 mL) and DIPEA (3.7 g, 29 mmol) and added to the previous solution prepared. The reaction mixture was stirred for 1.5 h at room temperature. DMF was removed under reduced pressure and the obtained residue was dissolved in 200 mL CH2Cl2, the solution was washed twice with 0.1 N HCl (200 mL) and followed by washing with water (200 mL). It was then dried over MgSO4 and CH2Cl2 was removed under vacuum to yield crude product. It was then purified by flash column chromatography to yield white solid product, FMOC-PEG-GFLG-MEDA-Z (6.2 g, 72%).
[0613]FMOC-PEG-GFLG-MEDA-Z (3.0 g, 3.0 mmol) was dissolved in CH2Cl2 (60 mL) of 0.2 M 2-Bromo-1,3,2-benzodioxaborole (2.4 g, 12 mmol). The reaction mixture was stirred overnight at room temperature. The reaction w...
example 2
Synthesis of CDP-Carbamate-S-S-Etoposide
[0617]
[0618]In a dry 100 mL round bottom flask, etoposide (1.0 g, 1.7 mmol) and TEA (2.5 g, 25 mmol) were dissolved in anhydrous THF (35 mL) under argon. To that solution, 4-nitrophenyl chloroformate (0.39 g, 1.95 mmol) in anhydrous THF (15 mL) was added dropwise over 30 min. The reaction mixture was stirred for additional 2 h at RT. The mixture was filtered and concentrated under reduced pressure to yield yellow solid. The solid was purified by flash column chromatography to yield light yellow solid (0.75 g, 59%).
[0619]In a dry 25 mL round bottom flask, 4-nitrophenyl carbonate ester of etoposide (100 mg, 0.13 mmol), 4-pyridylthiol cysteamine hydrochloride (35 mg, 0.16 mmol), DIPEA (34 mg, 0.27 mmol) were dissolved in DMF (5 mL). The reaction mixture was stirred at room temperature for 15 h. DMF was removed under reduced pressure to yield a light yellow solid. CH2Cl2 (25 mL) was added and it was washed with 0.1 N HCl (10 mL) twice. It was then...
example 4
CDP-PEG-SS-Tubulysin
Synthesis of CDP-PEG-SS-Py
[0623]A mixture of CDP-PEG (2 g, 0.43 mmole), which was synthesized according to a published procedure (Bioconjugate Chem. 2003, 14, 1007), pyridine dithioethylamine hydrochloric salt (384 mg, 1.73 mmole), EDC (333 mg, 1.73 mmole), and NHS (198 mg, 1.73 mmole) was dried overnight in a 200 mL round bottom flask under vacuum. Anhydrous DMF (40 mL) was then added, followed by DIEA (0.3 mL, 1.73 mmole). The reaction mixture was stirred under argon at room temperature for 4 h. Diethyl ether (300 mL) was then added into the mixture to precipitate the polymer. The crude product was dissolved in H2O (400 mL) and the solution was dialyzed using a 25K MWCO membrane (Spectra / Por 7) against water. The dialysis water was changed twice over a period of 24 h, after which the polymer containing solution was filtered through a 0.2 μm filter membrane and lyophilized to yield 1.64 g of CDP-PEG-SS-Py (82% yield) as a white solid.
Synthesis of CDP-PEG-SH
[0624...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com