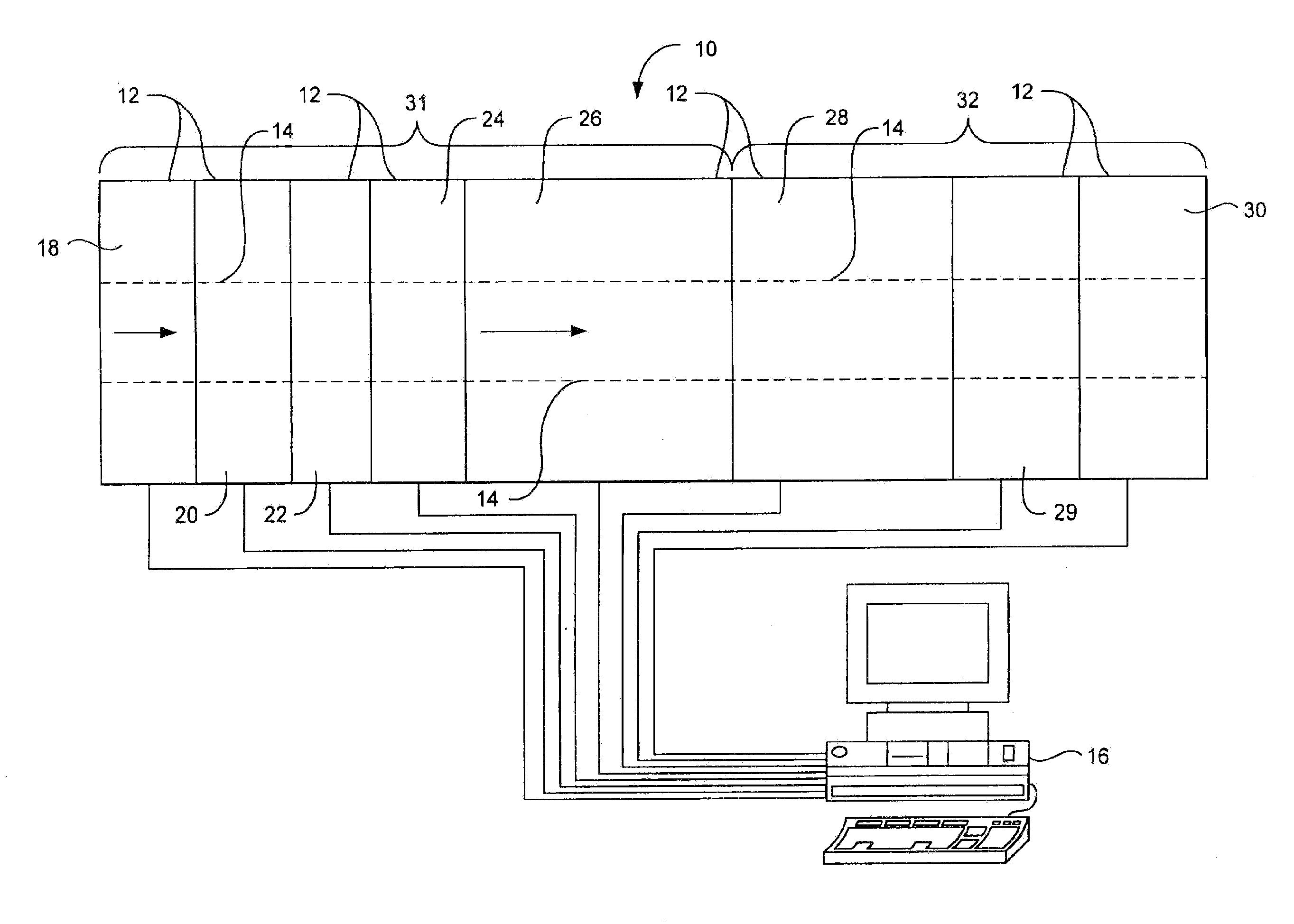
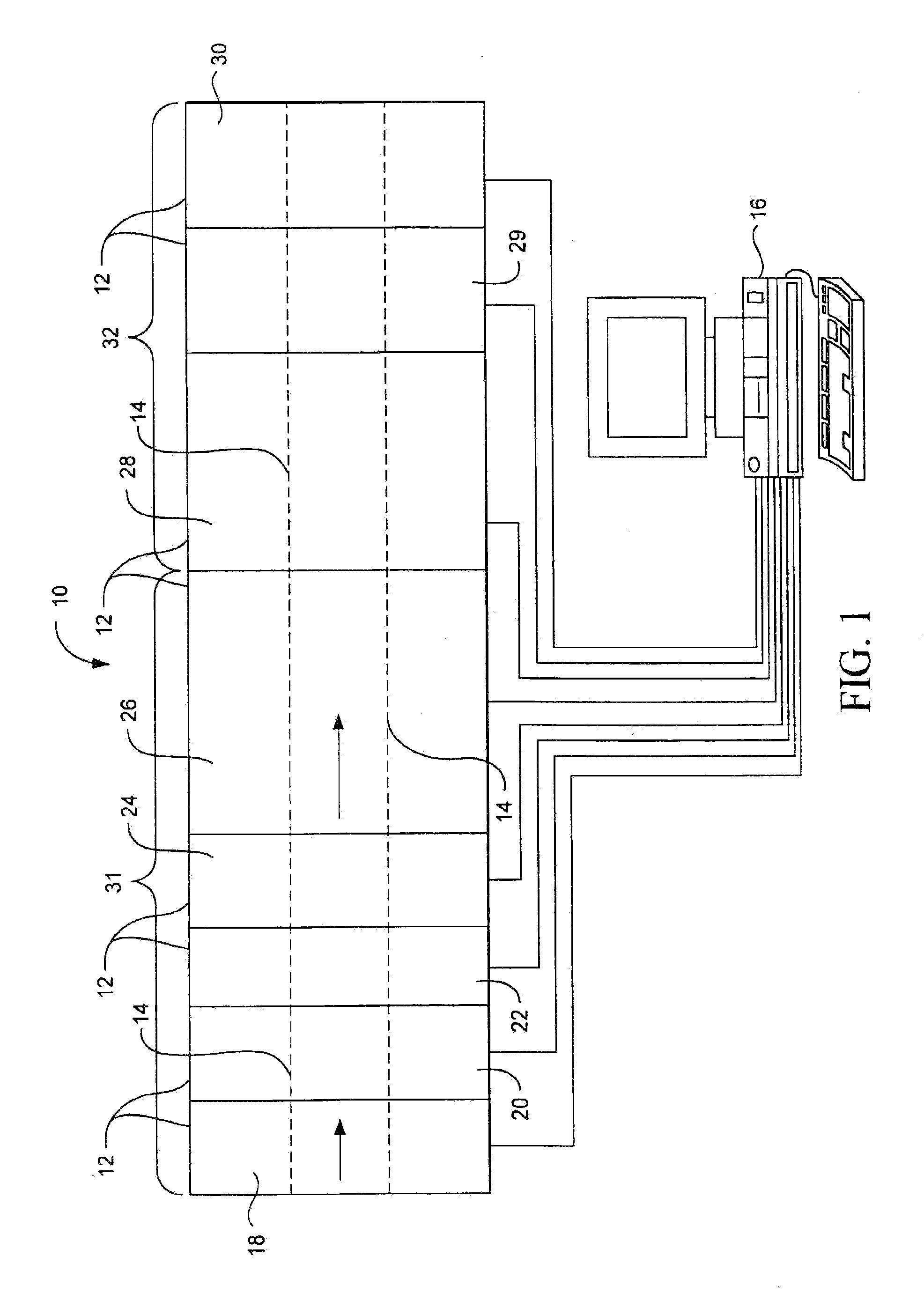
Method for screening microcrystallizations for crystal formation
a microcrystallization and crystallization technology, applied in the direction of burettes/pipettes, sequential/parallele process reactions, crystallization, etc., can solve the problems of large bottleneck, limited amount of highly purified proteins, time-consuming process of growing crystals with high diffraction quality, etc., to accelerate drug development, more bioactive, and dissolve faster
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0205]The system described above was used in a plurality of lysozyme crystallization experiments where lysozyme was crystallized in a mother liquor composition including 100 mM sodium acetate and 10% sodium chloride at a pH of 4.6. The volume of the hanging drop formed by the drop formation station was different for each experiment. FIGS. 10A-10D respectively illustrate crystal formed in hanging drops of 40 nL, 100 nL, 200 nL and 1000 nL. The crystals were formed regardless of the reduction in drop size. As a result, the system can be used with submicroliter hanging drop volumes.
example 2
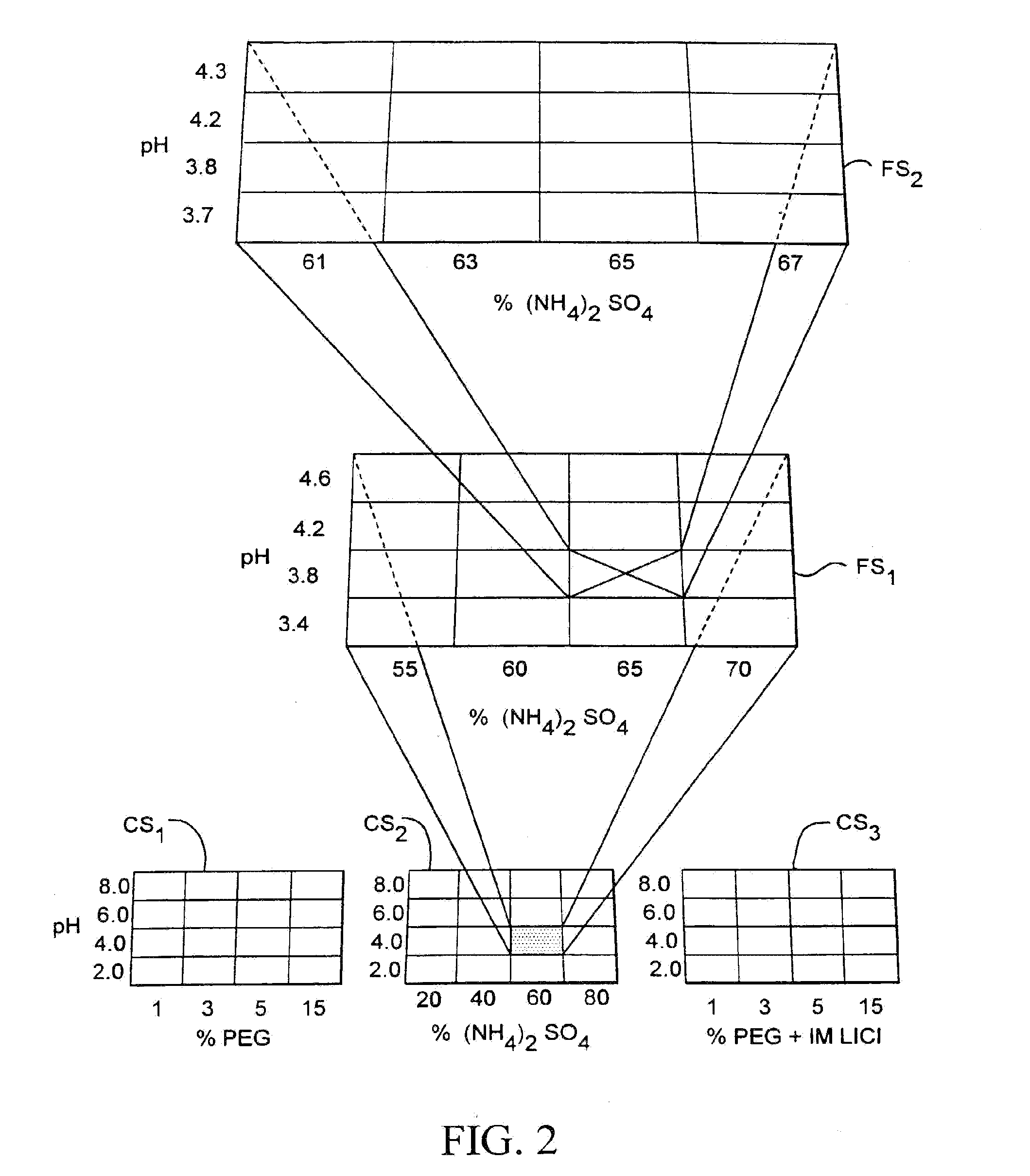
[0206]The system described above was used in a crystallization trial where the mother liquor for crystallizing lysozyme was optimized. During the coarse screen, 480 crystallization experiments were performed using each of the 480 mother liquors disclosed in FIG. 9. The results from each of the 480 experiments were compared to one another to identify one or more crystallization experiments yielding crystals with the most desirable characteristics. One of the identified coarse screen experiments was associated with a mother liquor composed of 30% MPD (.+-.2-methyl-2,4-pentanediol), 100 mM sodium acetate, 20 mM calcium chloride, at pH 4.6.
[0207]A fine screen consisting of 24 crystallization experiments was then performed. The composition of the mother liquors associated with each of the 24 crystallization experiments was selected relative to the composition of the mother liquor associated with the identified coarse screen experiment. The compositions of the 24 mother liquors selected f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com