Electrochemical Method and Apparatus For Removing Oxygen From a Compound or Metal
a compound or metal and oxygen technology, applied in the field of electrochemical methods and apparatus for removing oxygen from compounds or metals, can solve the problems of inability to use suitable materials for inert anodes, carbon anodes consumed, carbon contamination of electro-decomposition products, etc., to reduce or substantially eliminate the electronic reduce the electrical efficiency of the process, and increase the electro-conductive conductivity of the melt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037]A specific embodiment of the invention will now be described by way of example, with reference to the drawings, in which;
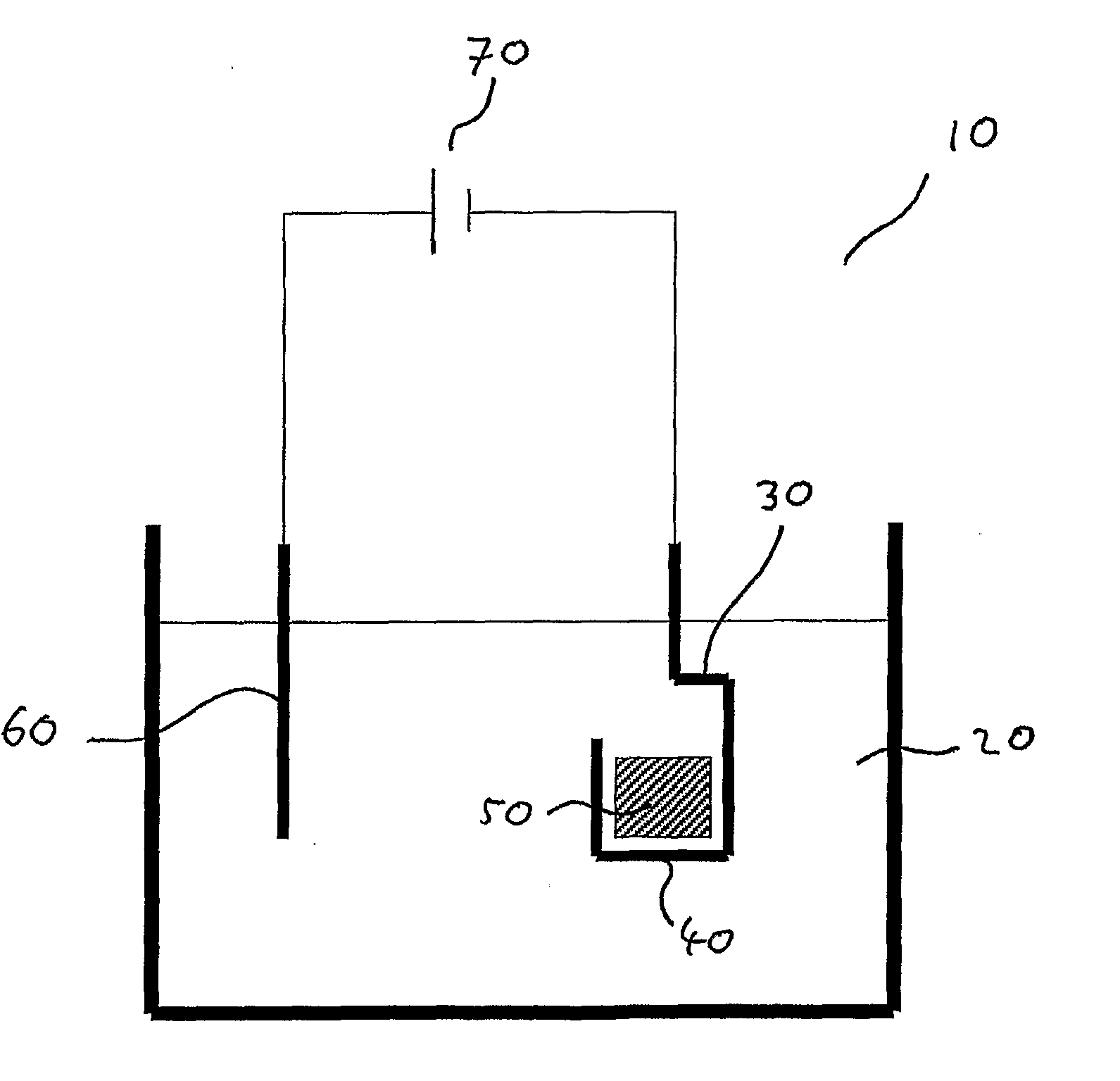
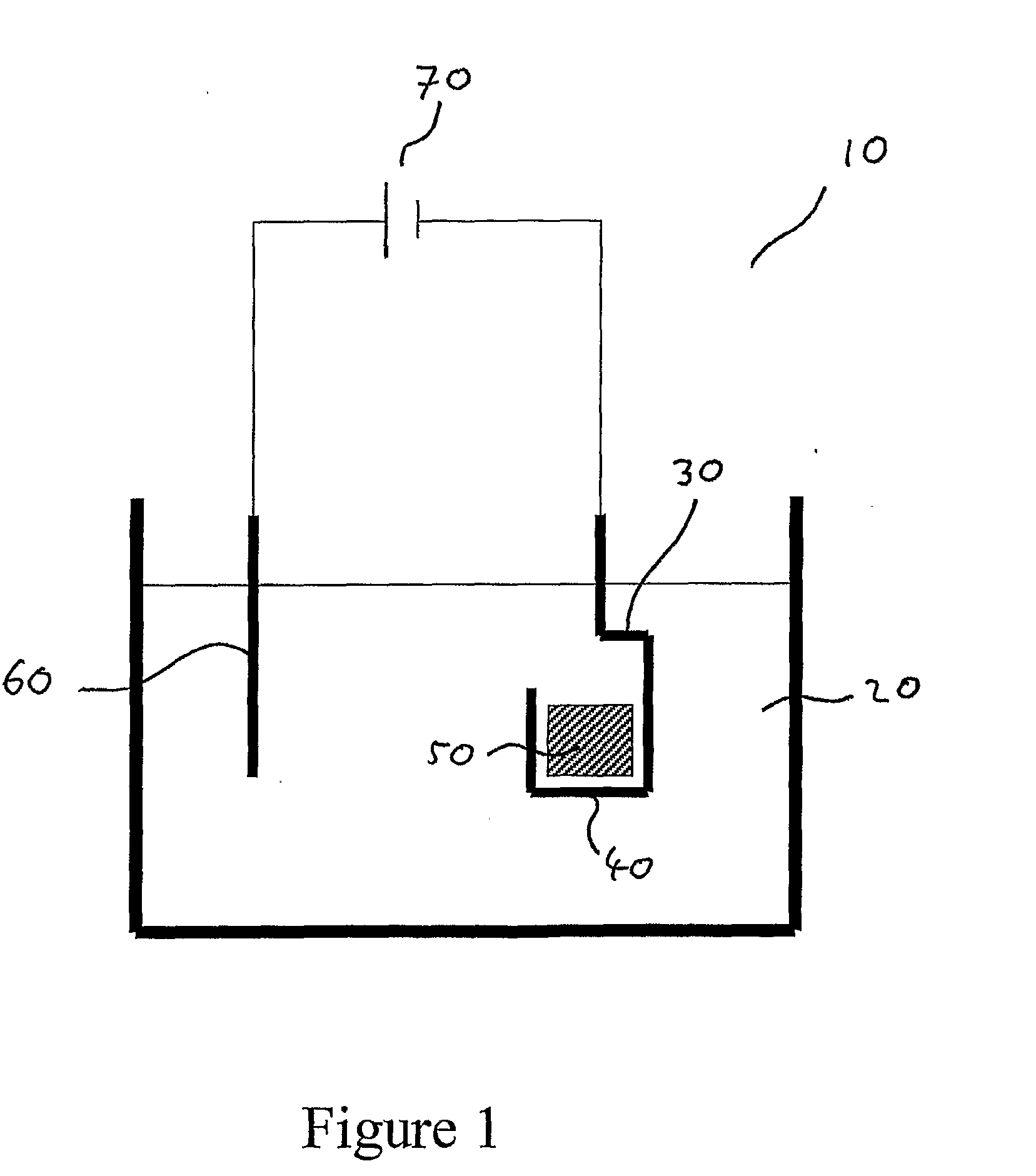
[0038]FIG. 1 shows a cell for an electro-decomposition process according to an embodiment of the invention.
[0039]FIG. 1 shows a cell 10 for electro-decomposition containing a melt 20 of composition 98% sodium hydroxide and 2% sodium oxide. A cathode 30 in the form of an iron basket 40 containing Fe2O3 particles 50 is immersed in the melt. An anode 60 of commercially pure nickel is also immersed in the melt, the anode and the cathode both being connected to a power supply 70.
[0040]In operation the melt is heated up to its operating temperature, for example 400° C. An operating potential of, for example, 2.5 to 3.0 V is applied between the anode and the cathode. At the operating potential, oxygen in the Fe2O3 transfers to the melt and is transported to the anode, where it is evolved as oxygen gas.
[0041]In a second embodiment, the melt is heated to an operating...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com