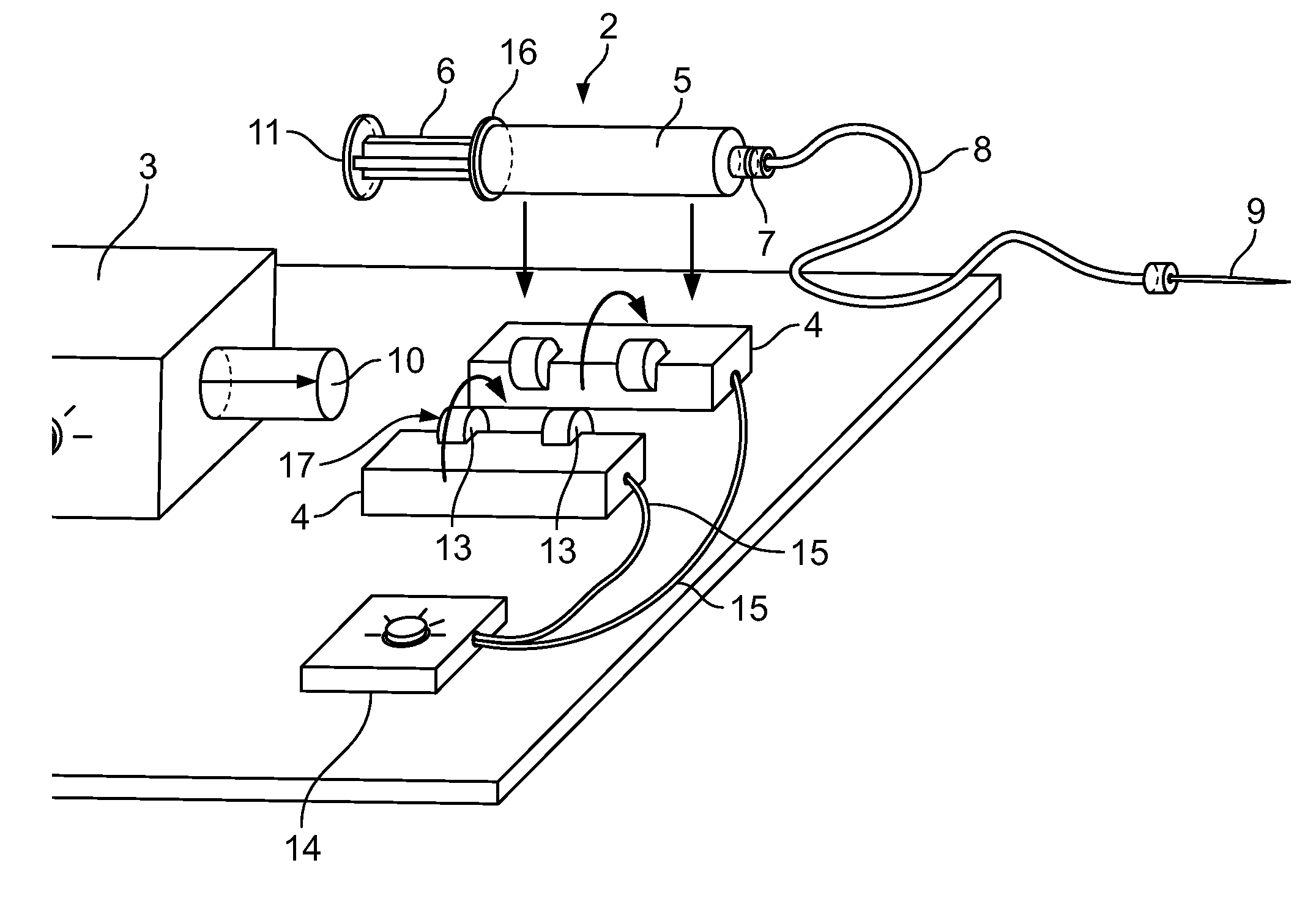
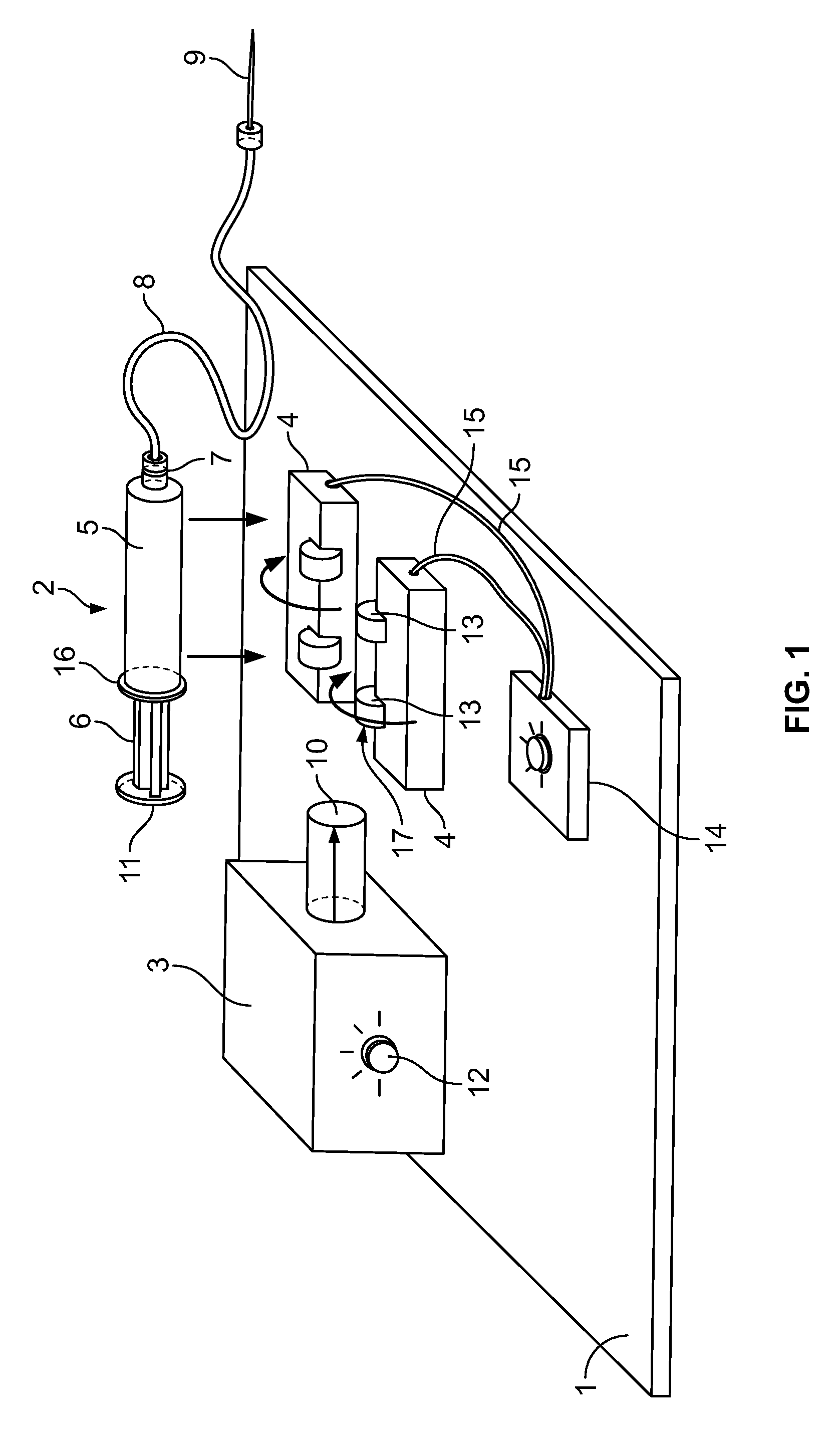
Automatic Liquid Injection System and Method
a liquid injection system and liquid injection technology, applied in the field of administration, can solve the problems of insufficient breakage or damage of particles, and disturb their distribution,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0033]A solution of gas filled microbubbles stabilised by a phospholipids interface was prepared according to Example 1 of U.S. Pat. No. 5,445,813. The dry matter concentration was 5 mg / ml in a saline solution (0.9% NaCl). Typically, the bubble size distribution extended from 0.2 to 15 μm. The concentration of bubbles between 2 and 5 μm was 5×107 microbubble / ml.
[0034]The solution was transferred in a 50 ml plastic syringe and samples were taken in time intervals for analysis. This represent the starting 100% of the bubble concentration. The syringe was mounted in the infusion unit and the elution started. The elution flow was fixed at 1.6 ml / min.
[0035]Aliquots of the eluted solution were analysed by Coulter measurement (bubbles distribution; size and concentration) and imaging.
TABLE 1RadiusVa1.00.1311.50.2942.00.5232.50.8173.01.1773.51.6024.02.0924.52.6485.03.2695.53.9556.04.7076.55.5247.06.4077.57.3558.08.3688.59.4469.010.5909.511.80010.013.07510.514.41511.015.82011.517.29112.018.8...
example 2
Preparation of Contrast Agents for Infusion
[0042]To test the efficiency of the present invention (a system of rotary syringe pump), different contrast agents for ultrasound echography were prepared.
[0043]Microbubble Suspensions
[0044]Phospholipid stabilised microbubbles were obtained in the following manner. 500 mg DAPC and 50 mg DPPA (Avanti Polar Lipids, Inc.) were dissolved in hexane / iso-propanol 8 / 2 (v / v) and dried in a round-bottomed flask using a rotary evaporator and, further, in a vacuum dessicator. After addition of water (100 ml), the suspension of lipids was heated at 75° C. for 1 hour under agitation and then extruded through a 0.8 μm polycarbonate filter (Nuclepore®). The resulting suspension and 10 g of poly-ethyleneglycol (Mw4000) were mixed and lyophilised. 2 g of the lyophilisate was introduced into a glass vial and sealed under SF6 or an air / C4F10 mixture. After reconstitution with 25 ml NaCl 0.9%, the resulting suspensions contained about 6×108 (SF6) or 1×109 (C4F1...
example 3
Determination of the Limit of Rotation Rate for the Syringe Used for Infusion
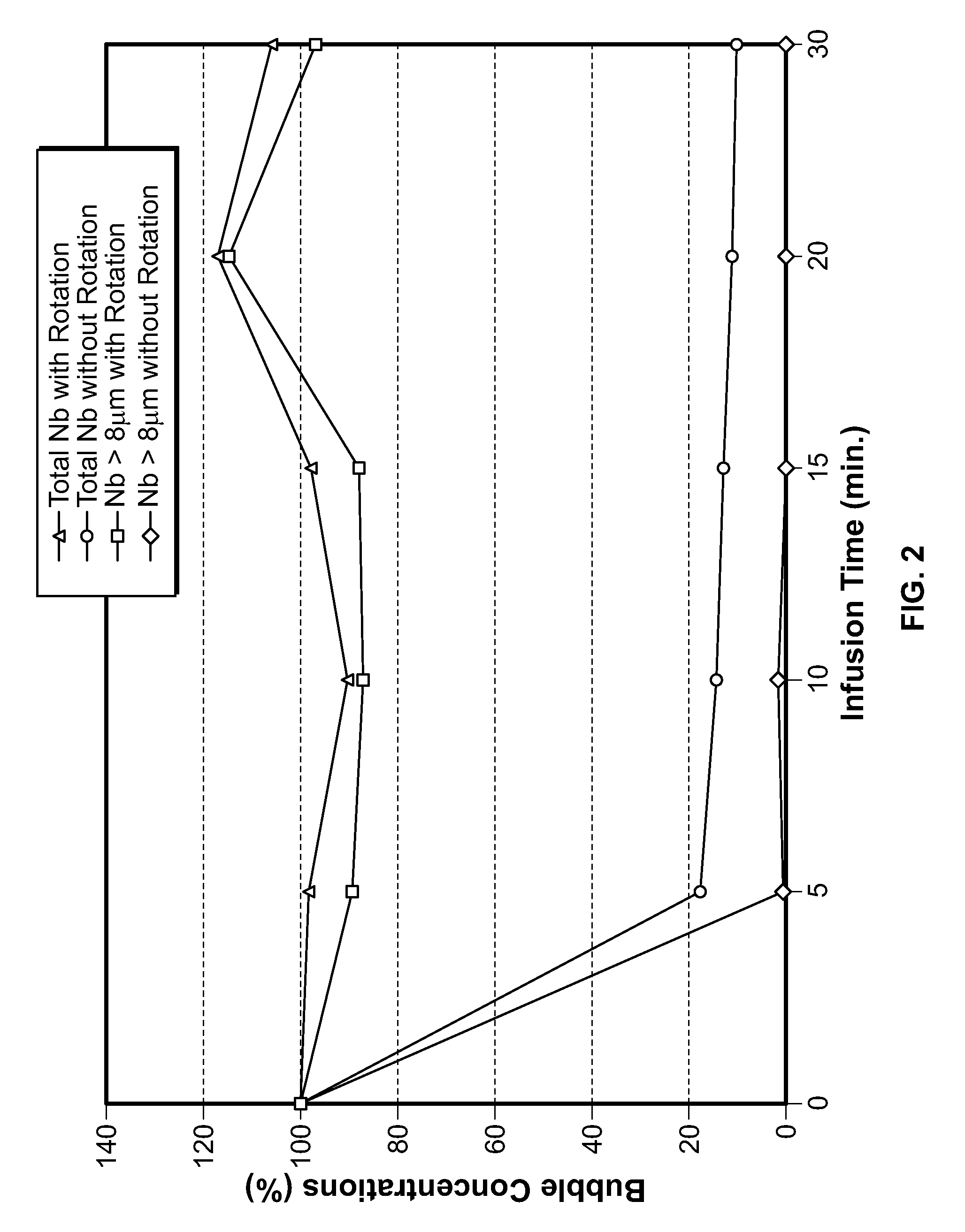
[0047]The effect of syringe rotation on stability of gas microbubble suspensions in the syringe used for infusion has been tested using a 50 ml syringe which was mounted on a rotation system which allows very low rotation speeds (about 1 rpm). Prior to its mounting the syringe was filled with 30 ml of phospholipid stabilised microbubble suspension. The mounted syringe was then rotated at different speeds: 0 (no rotation) 1.3, 2 and 60 rpm (1 Hz) and the suspension monitored taking one sample every 5 minutes. The samples were then analysed using Coulter counter. Table 3A shows the results obtained with a suspension of 3.1×108 microbubbles / ml having a mean diameter of 2.1 μm.
TABLE 3AHomogeneity of microbubble suspensions in the syringeas a function of the rotation rate and time (microbubbleconcentration 3.1 × 108 bubbles / ml)Syringe rotation ratesrpm01.326001.326001.3260Vrt011417652780114176527801142765278(min...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com