Method for recovering performance of electrolyzer for use in production of polysulfide and method for stopping holding electrolyzer
a technology of electrolyzer and polysulfide, which is applied in the direction of electrolysis process, electrolysis components, etc., can solve the problems of increasing the resistance of the membrane, increasing the electricity required, and increasing the voltag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
working example 1
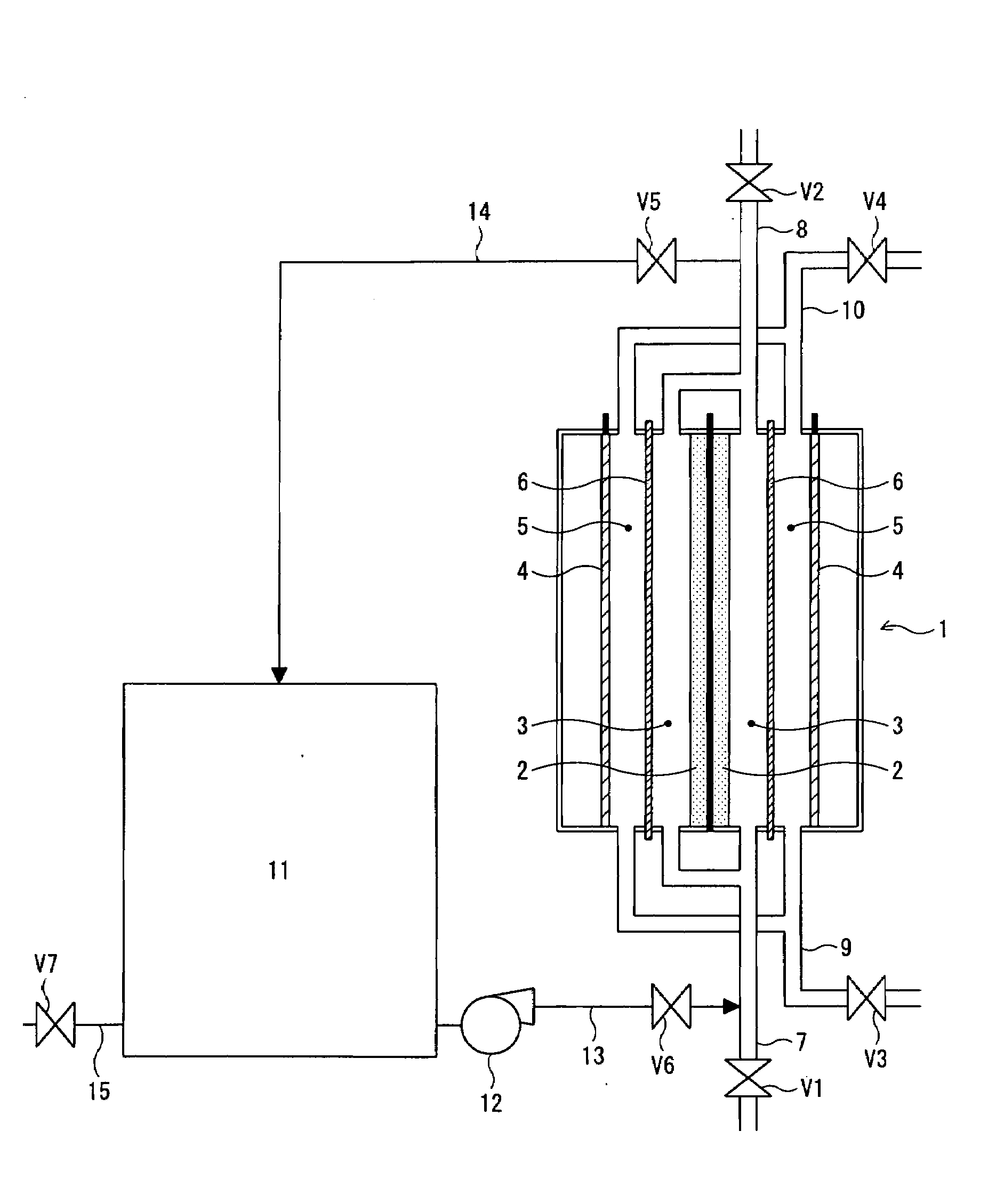
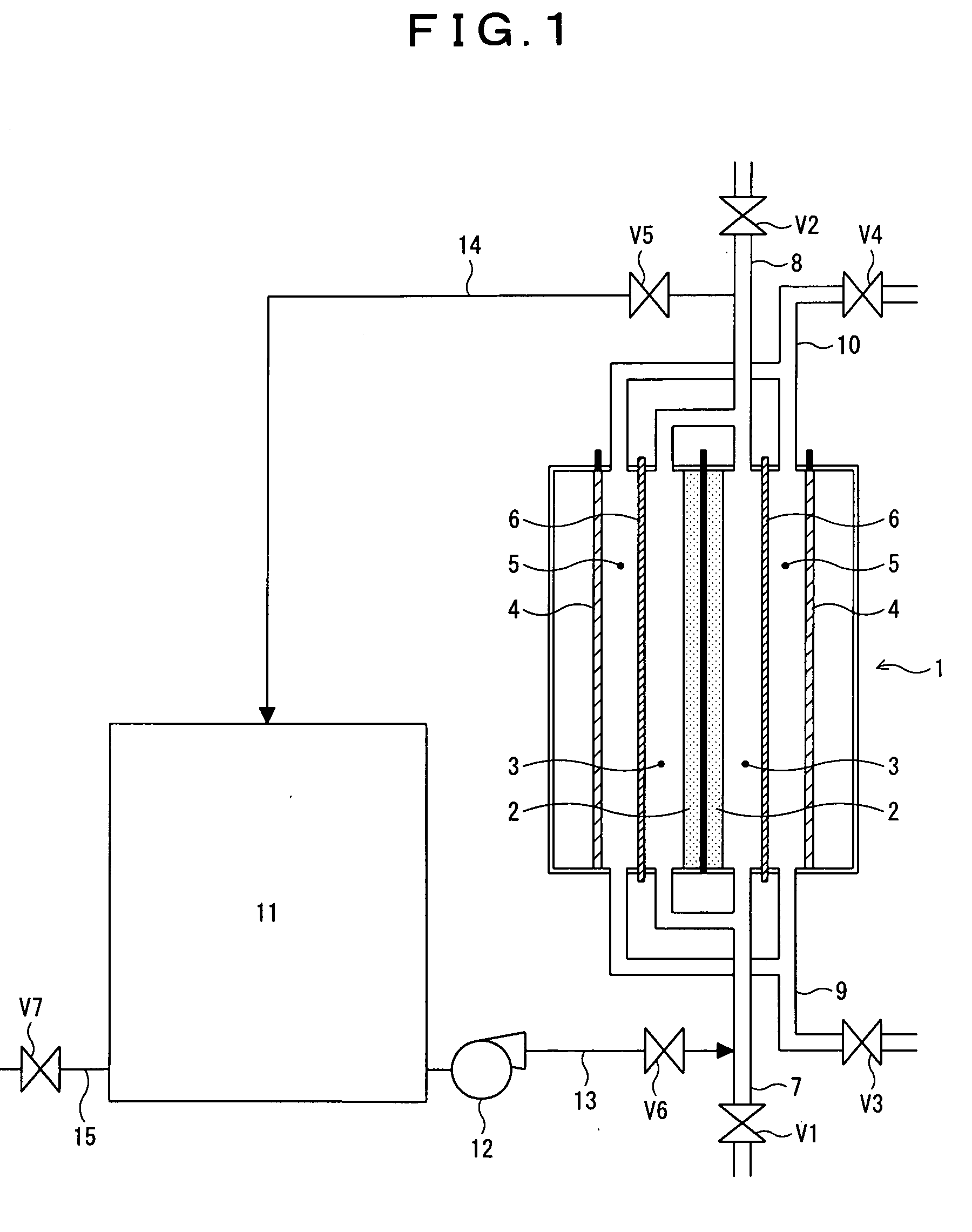
[0061]A white liquor having the following composition was oxidized by electrolytic oxidation. Electrolysis conditions were as follows: A double-chamber electrolyzer was assembled, the electrolyzer comprising a porous nickel member serving as an anode (an anode surface area per an anode compartment volume: 5600 m2 / m3, the average diameter of openings in the mesh of the anode: 0.51 mm, an anode surface area per a unit membrane area: 28 m2 / m2), iron expansion metal serving as a cathode, and a perfluoro resin cation-exchange membrane serving as a membrane. The white liquor having the following composition was introduced into the electrolyzer, and electrolysis was carried out under the condition of electrolysis temperature: 85° C., and current density at the membrane: 6 kA / m2, having thereby obtained a polysulfide liquor of polysulfide sulfur concentration 9 g / L at current efficiency 95%. Further, NaOH was formed on the cathode side of the electrolyzer at current efficiency 80%, and an a...
working example 2
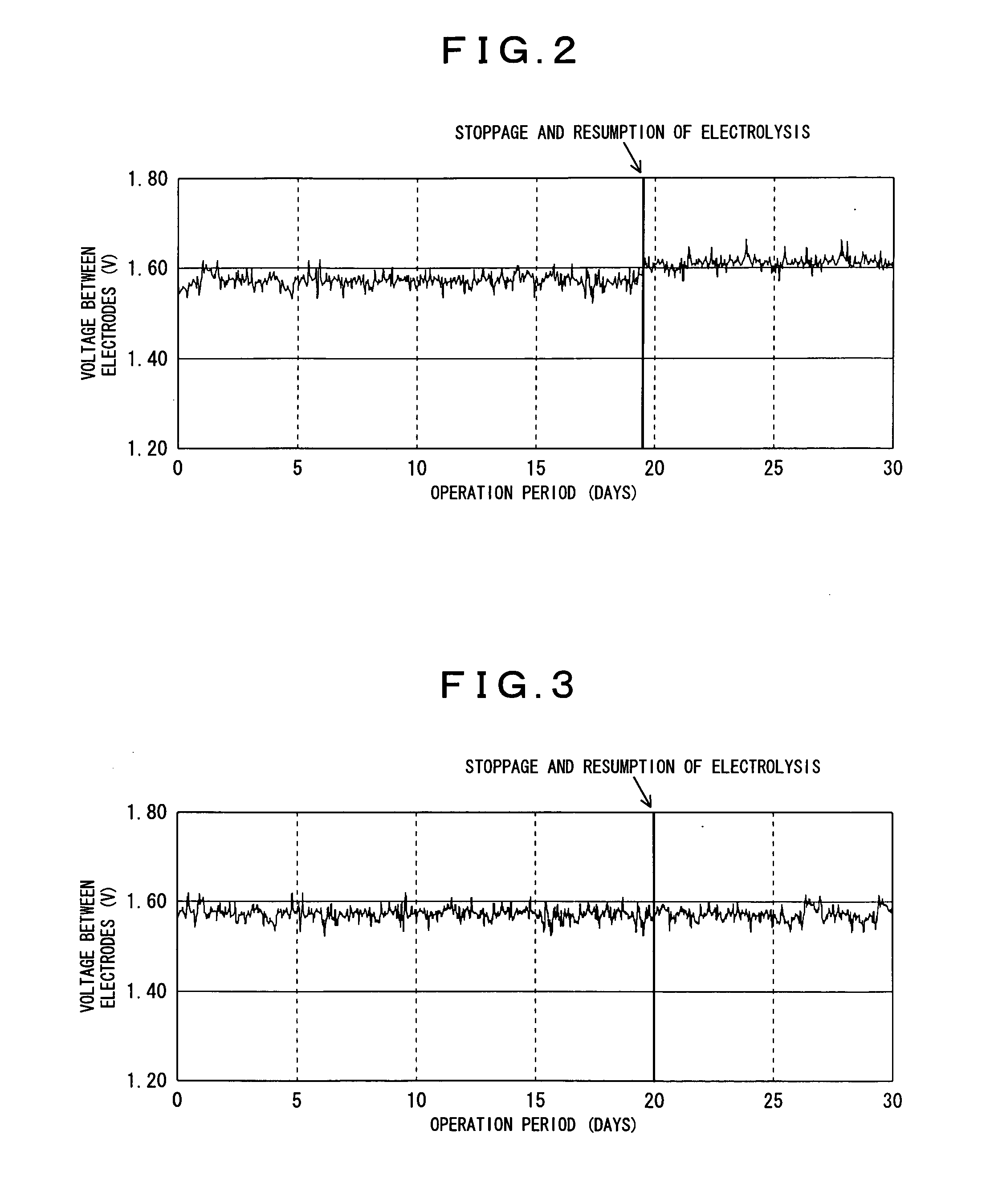
[0066]Electrolytic oxidation was conducted under a condition of the same electrolyzer, and white liquor as those for Working Example 1, except that the same hydrochloric acid that was used in Working Example 1, with 0.5 mass % of RESCOR A-825 (manufactured by Toei Kasei Co., Ltd.) as an anticorrosive, added thereto, was used as a cleaning fluid, and after stopping the electrolytic oxidation, cleaning was applied, subsequently, resuming electrolytic oxidation. Table 1 shows calcium concentration, and nickel concentration in the cleaning fluid at the time of completion of cleaning, and a voltage required for electrolytic oxidation 10 days after resumption of electrolysis.
working example 3
[0067]Electrolytic oxidation was conducted under a condition of the same electrolyzer, and white liquor as those for Working Example 1, except that 1.0 mass % of hydrochloric acid with 0.5 mass % of RESCOR A-825 (manufactured by Toei Kasei Co., Ltd.) as an anticorrosive, added thereto, was used as a cleaning fluid, and after stopping the electrolytic oxidation, cleaning was applied, subsequently, resuming electrolytic oxidation. Table 1 shows calcium concentration, and nickel concentration in the cleaning fluid at the time of completion of cleaning, and a voltage required for electrolytic oxidation 10 days after resumption of electrolysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com