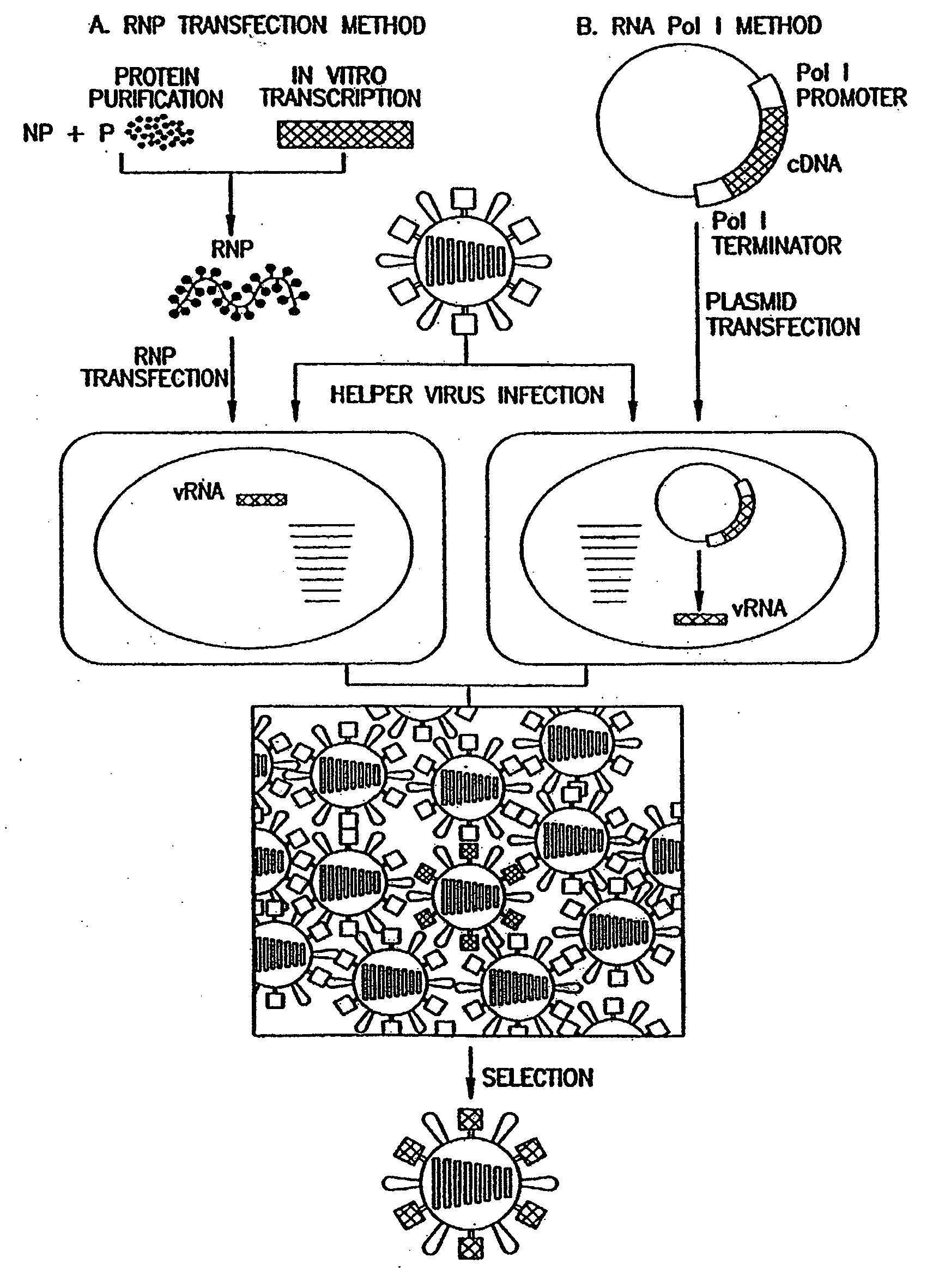
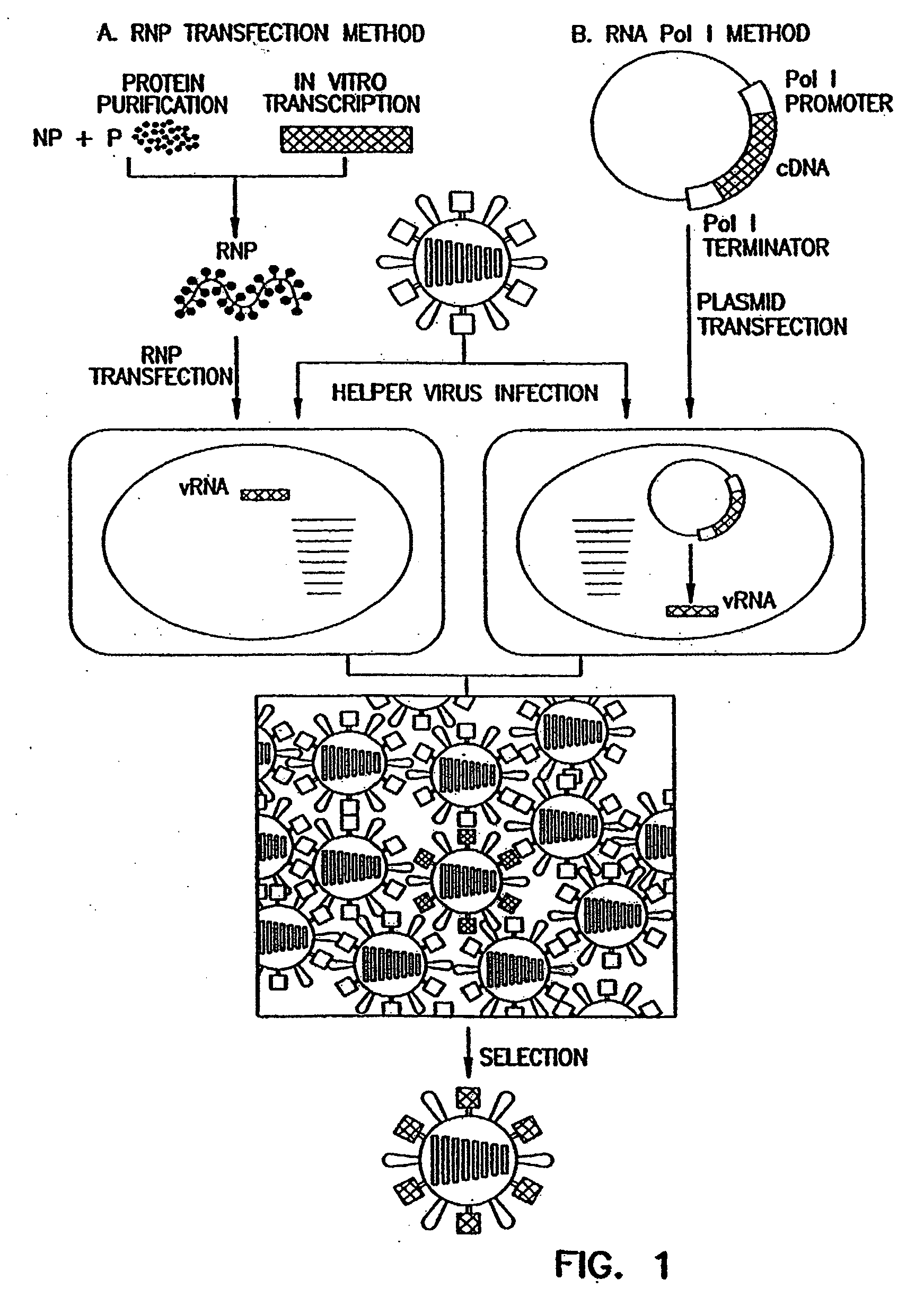
Recombinant influenza viruses for vaccines and gene therapy
a technology of recombinant influenza viruses and vaccines, applied in the direction of genetic material ingredients, antibody medical ingredients, peptide sources, etc., can solve the problems of generating segmented negative-sense rna viruses from cloned cdnas, limiting the progress of negative-sense rna viruses, and a formidable challenge, so as to enhance the efficiency of virus generation and enhance the effect of viruses as vaccine vectors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0055]Cells and viruses. 293T human embryonic kidney cells and Madin-Darby canine kidney cells (MDCK) were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum and in modified Eagle's medium (MEM) containing 5% newborn calf serum, respectively. All cells were maintained at 37° C. in 5% CO2. Influenza viruses A / WSN / 33 (H1N1) and A / PR / 8 / 34 (H1N1) were propagated in 10-day-old eggs.
[0056]Construction of plasmids. To generate RNA polymerase I constructs, cloned cDNAs derived from A / WSN / 33 or A / PR / 8 / 34 viral RNA were introduced between the promoter and terminator sequences of RNA polymerase I. Briefly, the cloned cDNAs were amplified by PCR with primers containing BsmBI sites, digested with BsmBI, and cloned into the BsmBI sites of the pHH21 vector which contains the human RNA polymerase I promoter and the mouse RNA polymerase I terminator, separated by BsmBI sites (FIG. 2). The PB2, PB1, PA, HA, NP, NA, M, and NS genes of the ...
example 2
[0071]Expression of the influenza virus proteins PB2, PB1, PA, and NP leads to replication and transcription of an artificial viral RNA. To generate influenza VLPs, the RNA polymerase I system for the intracellular synthesis of influenza viral RNAs in vivo was employed (FIG. 7). In this system, a cDNA encoding a reporter gene in antisense orientation is flanked by the 5′ and 3′ noncoding regions of an influenza viral RNA. This cassette is inserted between an RNA polymerase I promoter and terminator. Transfection of such constructs into eukaryotic cells leads to transcription of the reporter gene by cellular RNA polymerase I, thereby generating influenza virus-like RNAs (Neumann et al., 1994). Upon influenza virus infection, the artificial vRNAs are replicated and transcribed by the viral polymerase complex, resulting in the expression of the reporter gene.
[0072]To determine whether expression of the PB2, PB1, PA, and NP proteins leads to expression of the reporter gene encoded by th...
example 3
[0084]By using the Cre-loxP system, one can generate packaging cell lines for the production of replication-defective viruses. For example, a protein expression vector is prepared that contains a transcription stop cassette (e.g., pBS302 of Life Technologies, Bethesda, Md.; and Sauer et al., 1993; Lasko et al., 1992; Pichel et al., 1993; Bolivar et al., 1977; Stuhl et al., 1981; Stuhl, 1985; Fiers et al., 1978), flanked by two loxP sites, and one of the viral genes. Transcription, initiated at the promoter sequence, is blocked at the transcription stop sites. Thus, the viral gene is not transcribed and translated. A cell that is stably transfected with such a vector is infected with an influenza virus that lacks the vRNA encoding the gene cloned into the loxP system. This virus also contains an additional vRNA encoding the Cre protein. This virus is not viable in normal cells, because it lacks one of its vRNAs. However, in the packaging cell line, the Cre protein which is expressed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com