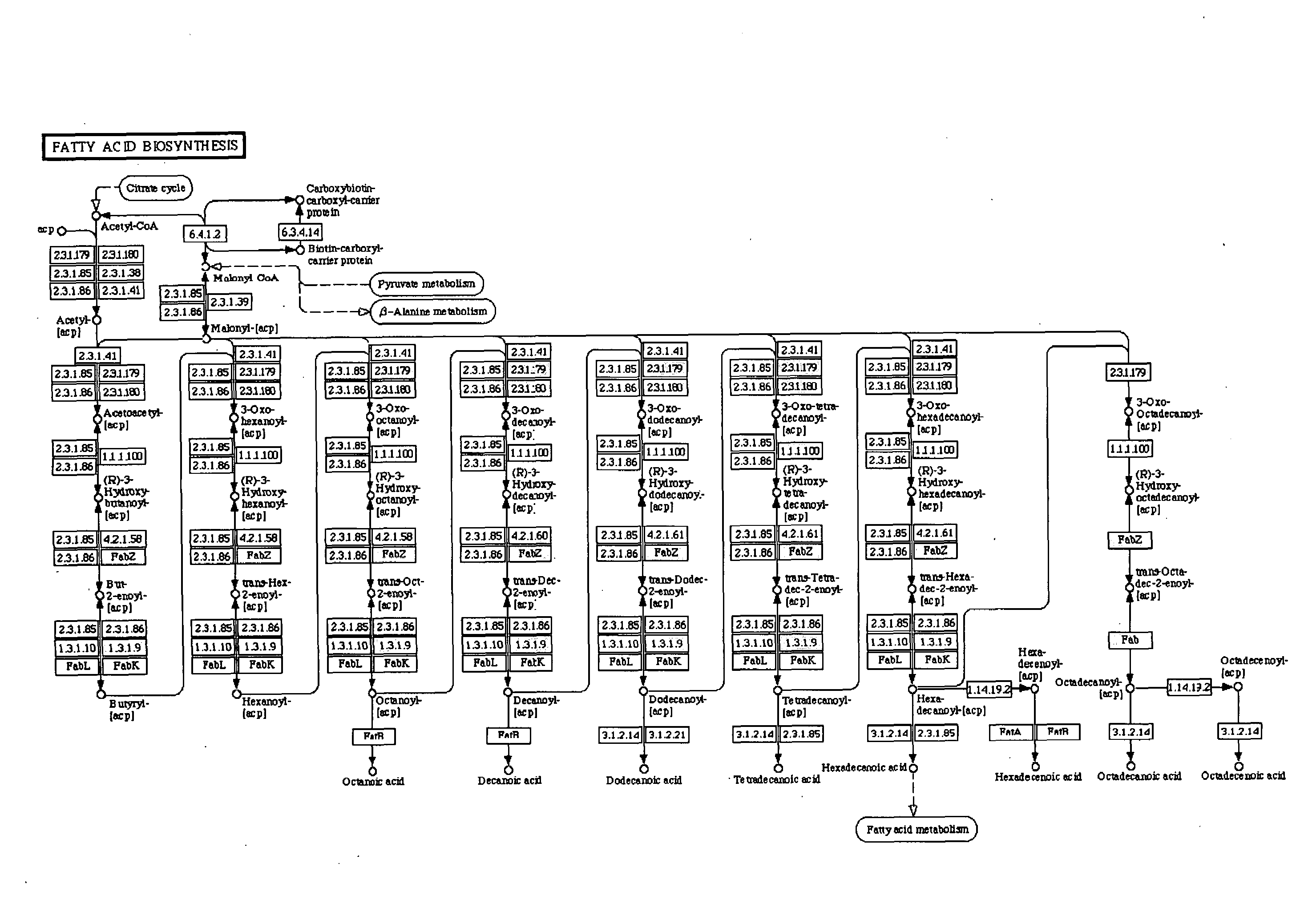
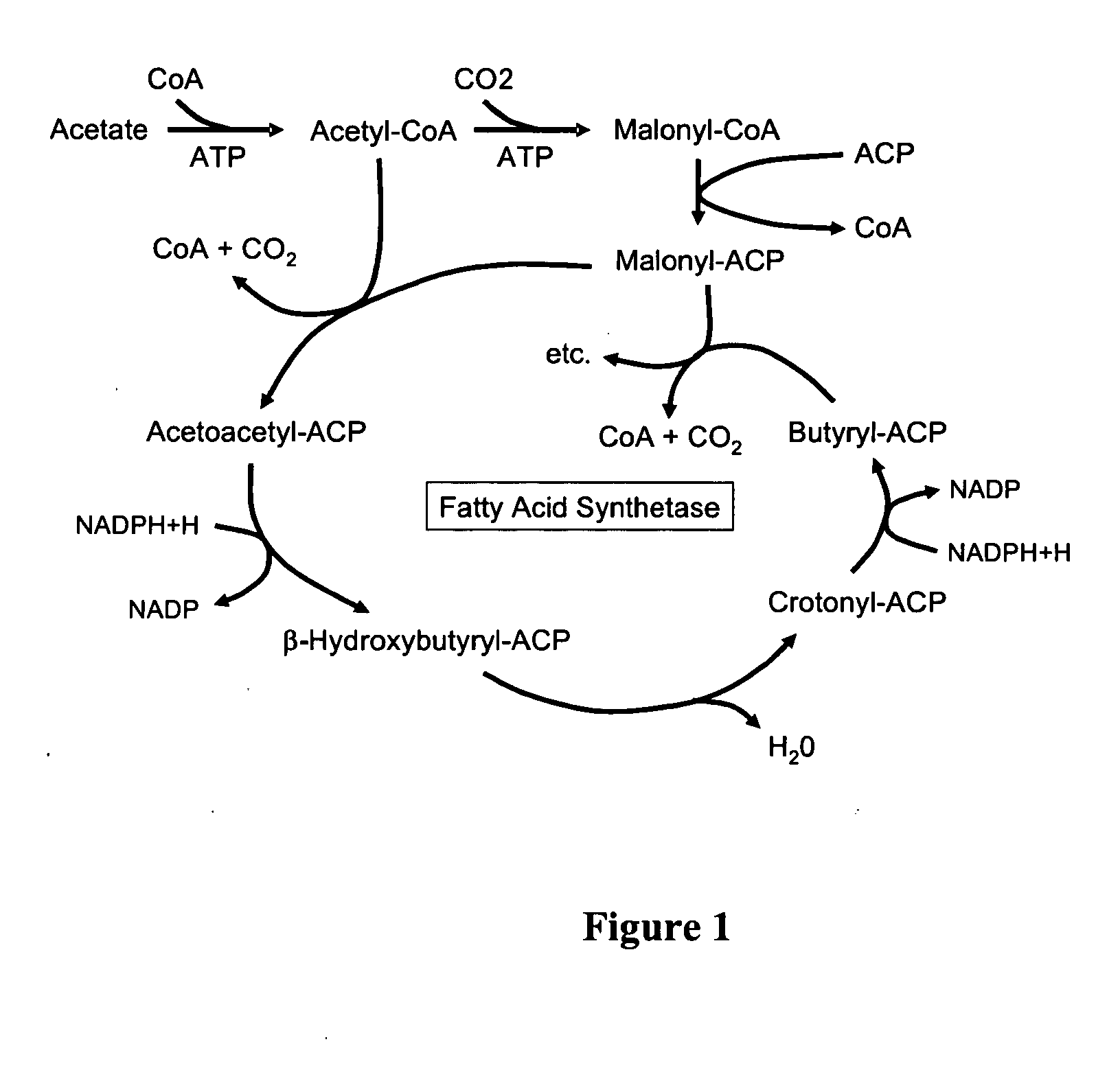
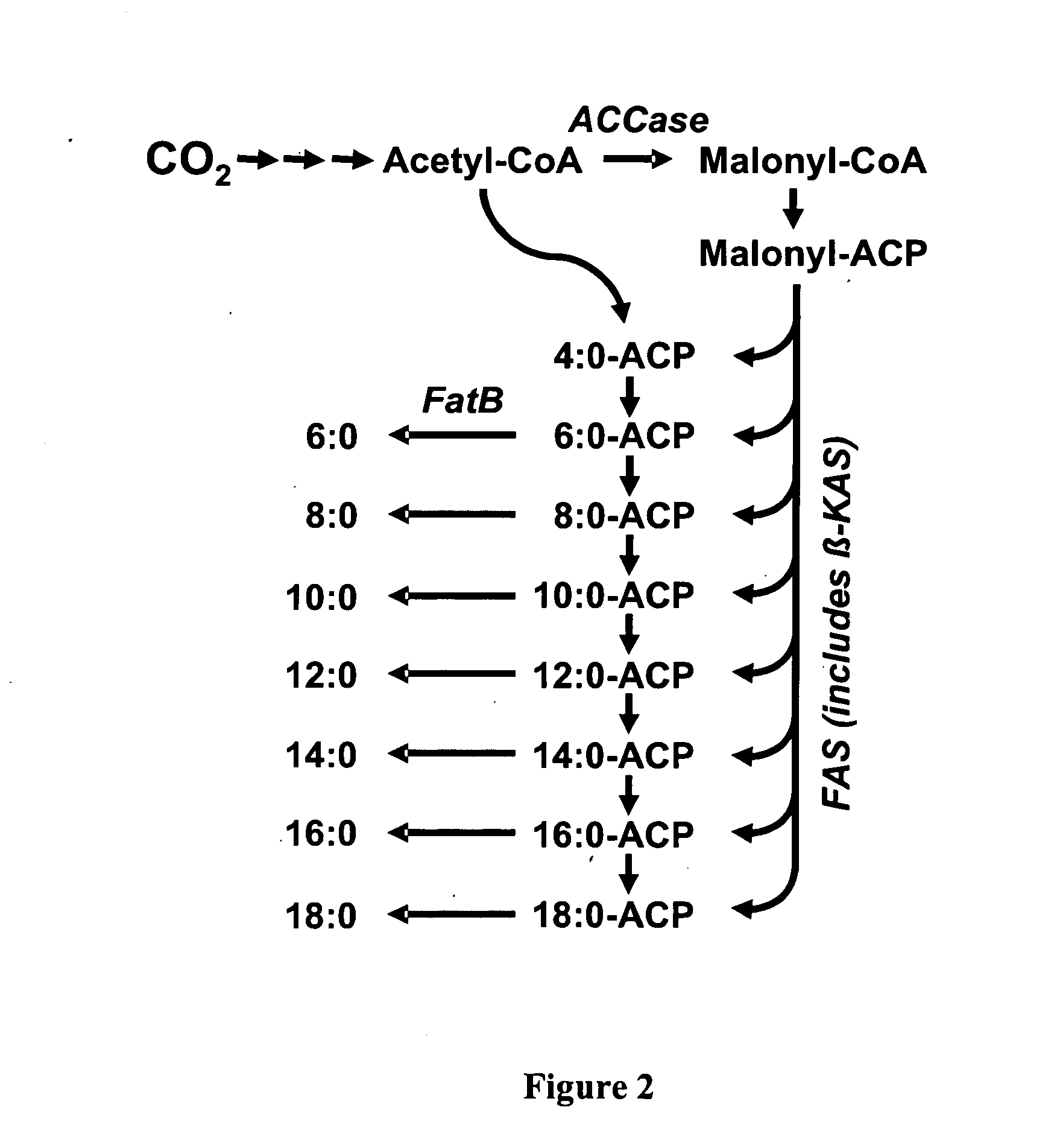
Secretion of fatty acids by photosynthetic microorganisms
a technology of photosynthetic microorganisms and fatty acids, which is applied in the field of photosynthetic microorganisms, can solve the problems of no disclosure of converting inorganic carbon directly into secreted fatty acids, cost-intensive steps in the accumulation of neutral lipids, and no cost reduction in the production of lipid-based products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Secretion of Fatty Acids by Strains Derived from the Unicellular Photoautotrophic Cyanobacterium Synechococcus elongatus PCC 7942
[0076]The Cuphea hookeriana FatB2 gene encoding an acyl-ACP thioesterase (ChFatB2) enzyme was modified for optimized expression in Synechococcus elongatus PCC 7942. First, the portion of the gene that encodes the plastid transit peptide region of the native ChFatB2 protein was removed. The remainder of the coding region was then codon-optimized using the “Gene Designer” software program (version 1.1.4.1) provided by DNA2.0, Inc. The nucleotide sequence of this derivative of the ChFatB2 gene (hereafter ChFatB2-7942) is provided as SEQ ID NO:7. The protein sequence encoded by this gene is provided in SEQ ID NO:8.
[0077]Two different versions of the trc promoter, trc (Egon, A., et al., Gene (1983) 25:167-178) and “enhanced trc” (hereafter trcE, from pTrcHis A, Invitrogen) were used to drive the expression of ChFatB2-7942 in S. elongatus PCC 7942. The trc promo...
example 2
Secretion of Fatty Acids by Strains Derived from the Unicellular Photoheterotrophic Cyanobacterium Synechocystis sp. PCC 6803
[0082]The trcE::ChFatB2-7942 and trc::ChFatB2-7942 fusion fragments, together with the lacIq gene, were cloned into the shuttle vector pSGI-YC03 (SEQ ID NO:11), which enables transformation of Synechocystis sp. PCC 6803 via double homologous recombination-mediated integration into the “RS1” site of the chromosome (Williams, Methods Enzymol. (1988) 167:766-778). The constructed plasmid containing the trcE::ChFatB2-7942 expression cassette and lacIq gene is designated pSGI-YC08. SEQ ID NO:12 represents the sequence between and including the RS1 recombination sites of pSGI-YC08. The constructed plasmid containing the trc::ChFatB2-7942 expression cassette and lacIq gene is designated pSGI-YC14. SEQ ID NO:13 represents the sequence between and including the RS1 recombination sites of pSGI-YC14.
[0083]Each of the plasmids pSGI-YC08, pSGI1-YC14, and the control vector...
example 3
Secretion of Fatty Acids by Strains Derived from the Filamentous Cyanobacterium Anabaena variabilis ATCC 29413
[0085]The trc::ChFatB2-7942 and trcE::ChFatB2-7942 fusion fragments, together with the lacIq gene, were PCR amplified using primers RS3-3F (SEQ ID NO:14) and 4YC-rrnBter-3 (SEQ ID NO:15) from pSG1-YC14 and pSGI-YC08, respectively, and then cloned into the shuttle vector pEL17, which enables transformation of A. variabilis ATCC 29413 via double homologous recombination-mediated integration into the nifU1 locus of the chromosome (Lyons and Thiel, J. Bacteriol. (1995) 177:1570-1575). The constructed plasmids are designated pSG1-YC69 and pSG1-YC70 for trc::ChFatB2-7942 and trcE::ChFatB2-7942, respectively.
[0086]Each of the plasmids pSG1-YC69, pSG1-YC70, along with the control vector pEL17, are introduced into wild-type A. variabilis ATCC 29413 cells via tri-parental conjugation, as described by Elhai and Wolk (Methods Enzymol. (1988) 167:747-754). Both recombinant and control st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameters | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com