Rnase h-based assays utilizing modified RNA monomers
a technology of rnase and assays, which is applied in the direction of biochemistry, organic chemistry, biochemical apparatus and processes, etc., can solve the problems of non-specific amplification, false positives in certain assays, and the reaction mixture cannot support primer extension at lower temperatures, so as to achieve greatly enhanced specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning of Codon Optimized RNase H2 Enzymes from Thermophilic Organisms
[0217]This example describes the cloning of codon optimized RNase H2 enzymes from thermophilic organisms.
[0218]To search for functional novel RNase H2 enzymes with potentially new and useful activities, candidate genes were identified from public nucleotide sequence repositories from Archaeal hyperthermophilic organisms whose genome sequences had previously been determined. While RNase H2 enzymes do share some amino acid homology and have several highly conserved residues present, the actual homology between the identified candidate genes was low and it was uncertain if these represented functional RNase H2 enzymes or were genes of unknown function or were non-functional RNase H2 genes. As shown in Table 4, five genes were selected for study, including two organisms for which the RNase H2 genes have not been characterized and three organisms to use as positive controls where the RNase H2 genes (rnhb) and function...
example 2
Expression of Recombinant RNase H2 Peptides
[0222]The following example demonstrates the expression of recombinant RNase H2 peptides.
[0223]The five synthetic gene sequences from Example 1 were subcloned using unique Bam HI and Hind III restriction sites into the bacterial expression vector pET-27b(+) (Novagen, EMD Biosciences, La Jolla, Calif.). This vector places six histidine residues (which together comprise a “His-tag”) (SEQ ID NO: 292) at the carboxy terminus of the expressed peptide (followed by a stop codon). A “His-tag” permits use of rapid, simple purification of recombinant proteins using Ni affinity chromatography, methods which are well known to those with skill in the art. Alternatively, the synthetic genes could be expressed in native form without the His-tag and purified using size exclusion chromatography, anion-exchange chromatography, or other such methods, which are also well known to a person of ordinary skill in the art.
[0224]BL21(DE3) competent cells (Novagen) w...
example 3
RNase H2 Activity for the Recombinant Peptides
[0230]The following example demonstrates RNase H2 activity for the recombinant peptides.
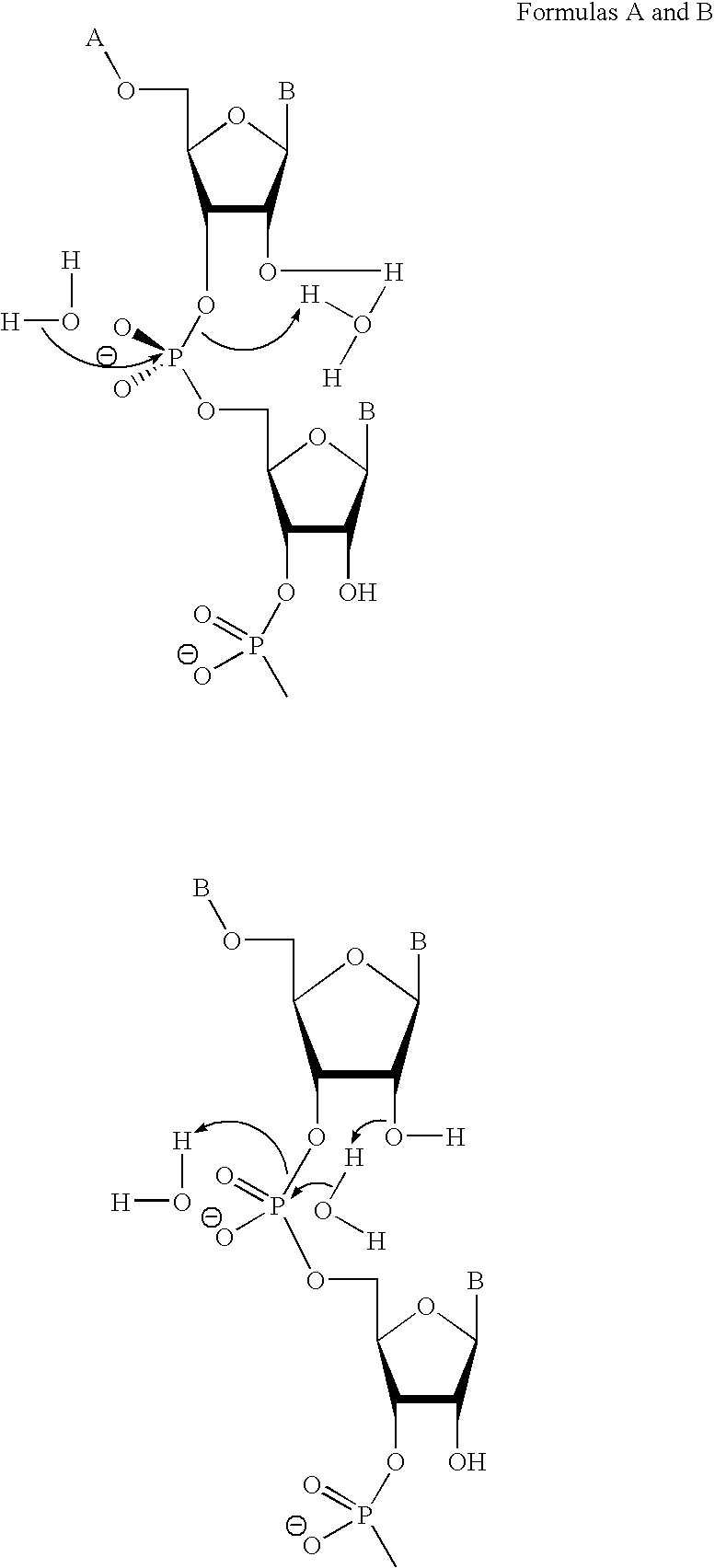
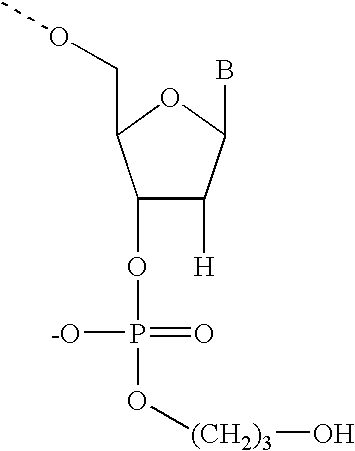
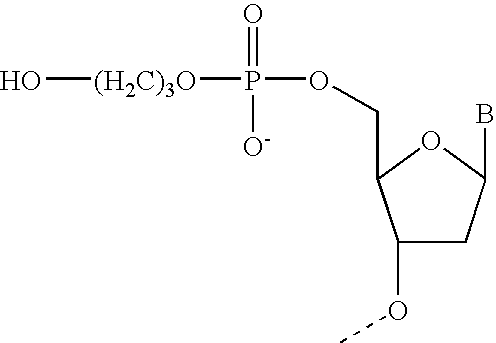
[0231]RNase H enzymes cleave RNA residues in an RNA / DNA heteroduplex. All RNase H enzymes can cleave substrates of this kind when at least 4 sequential RNA residues are present. RNase H1 enzymes rapidly lose activity as the RNA “window” of a chimeric RNA / DNA species is shortened to less than 4 residues. RNase H2 enzymes, on the other hand, are capable of cleaving an RNA / DNA heteroduplex containing only a single RNA residue. In all cases, the cleavage products contain a 3′-hydroxyl and a 5′-phosphate (see FIG. 1). When multiple RNA residues are present, cleavage occurs between RNA bases, cleaving an RNA-phosphate linkage. When only a single RNA residue is present, cleavage occurs only with Type II RNase H enzymes. In this case cleavage occurs on the 5′-side of the RNA base at a DNA-phosphate linkage (see FIG. 3). RNase H enzymes require the presence of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com