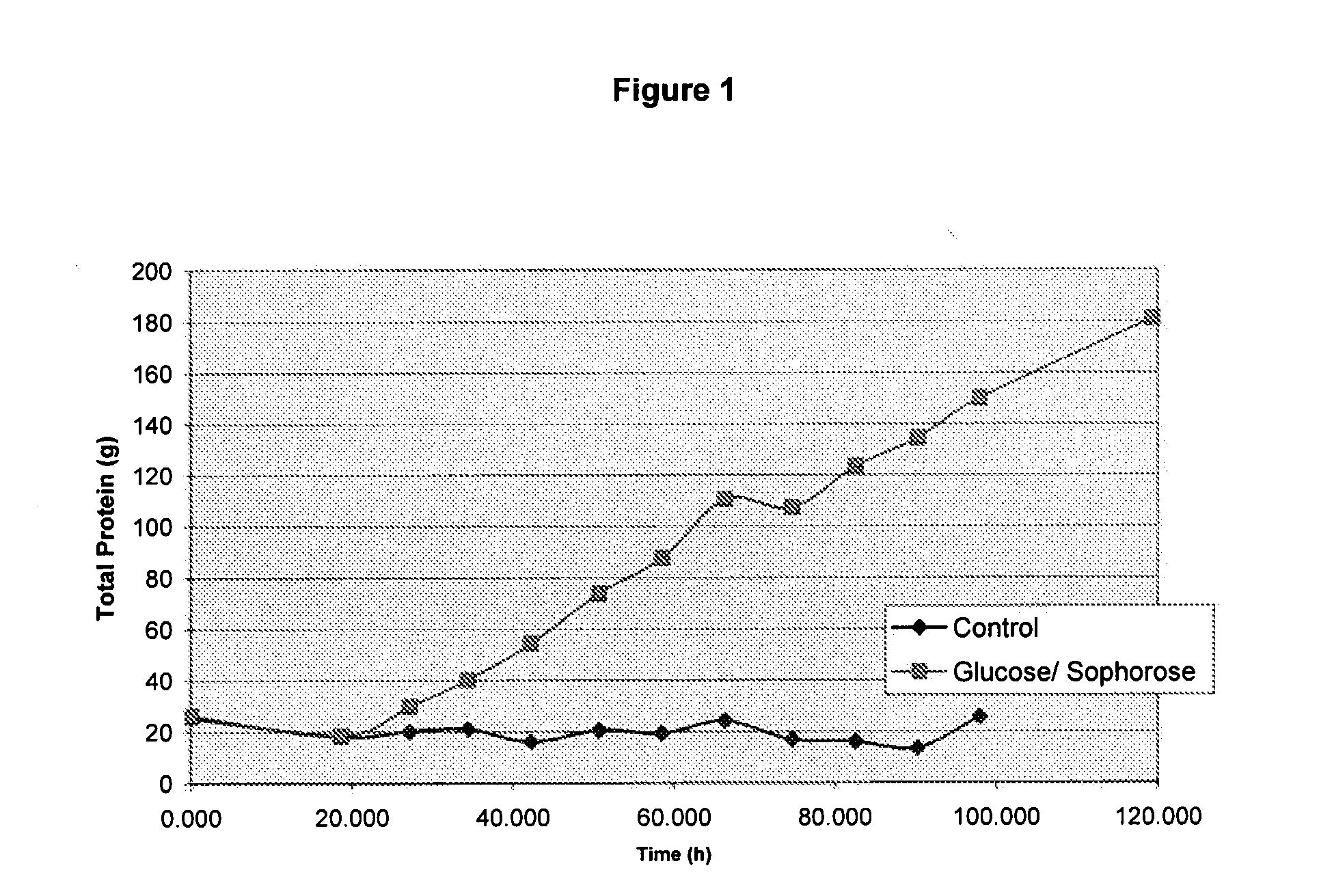
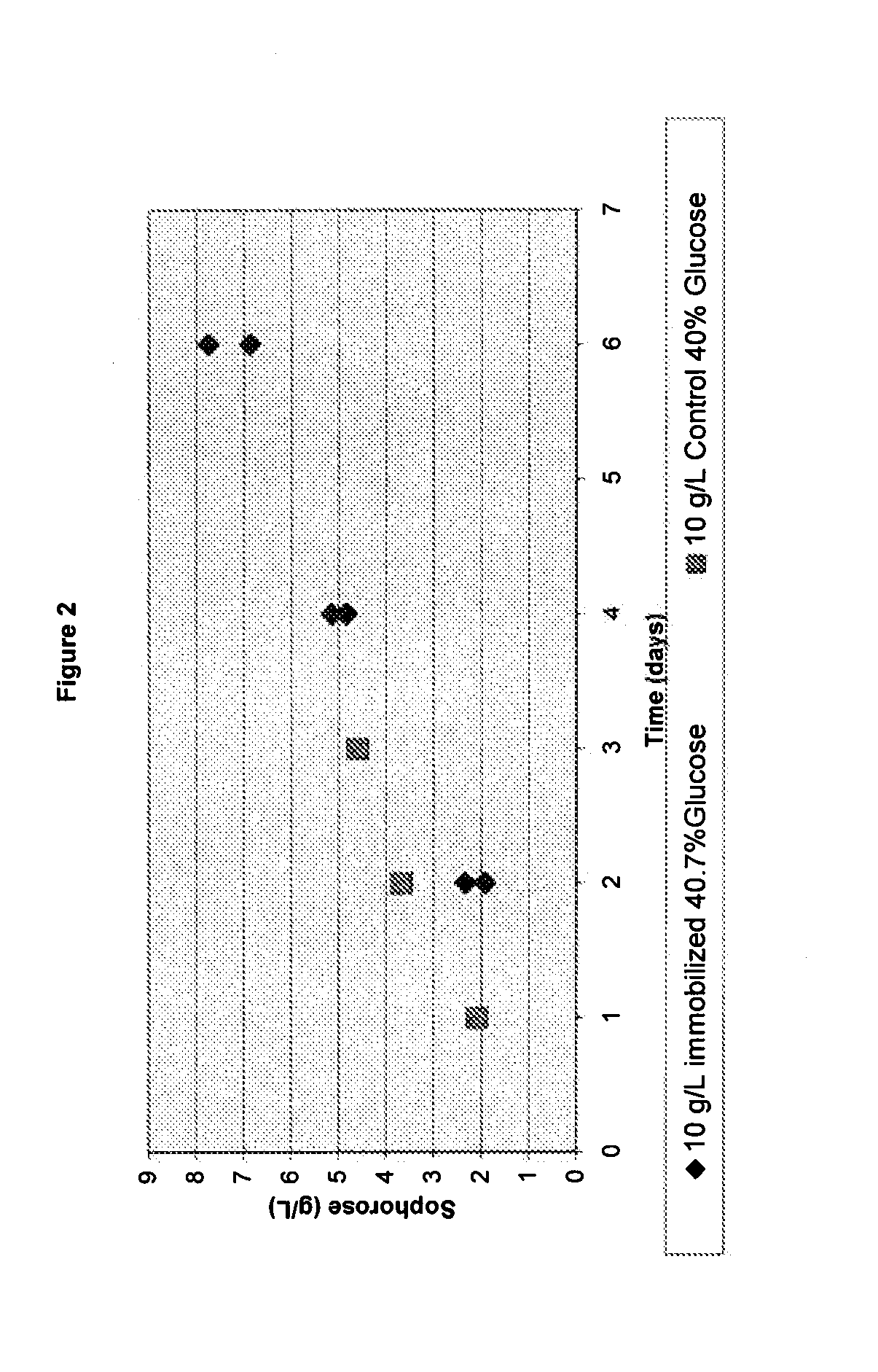
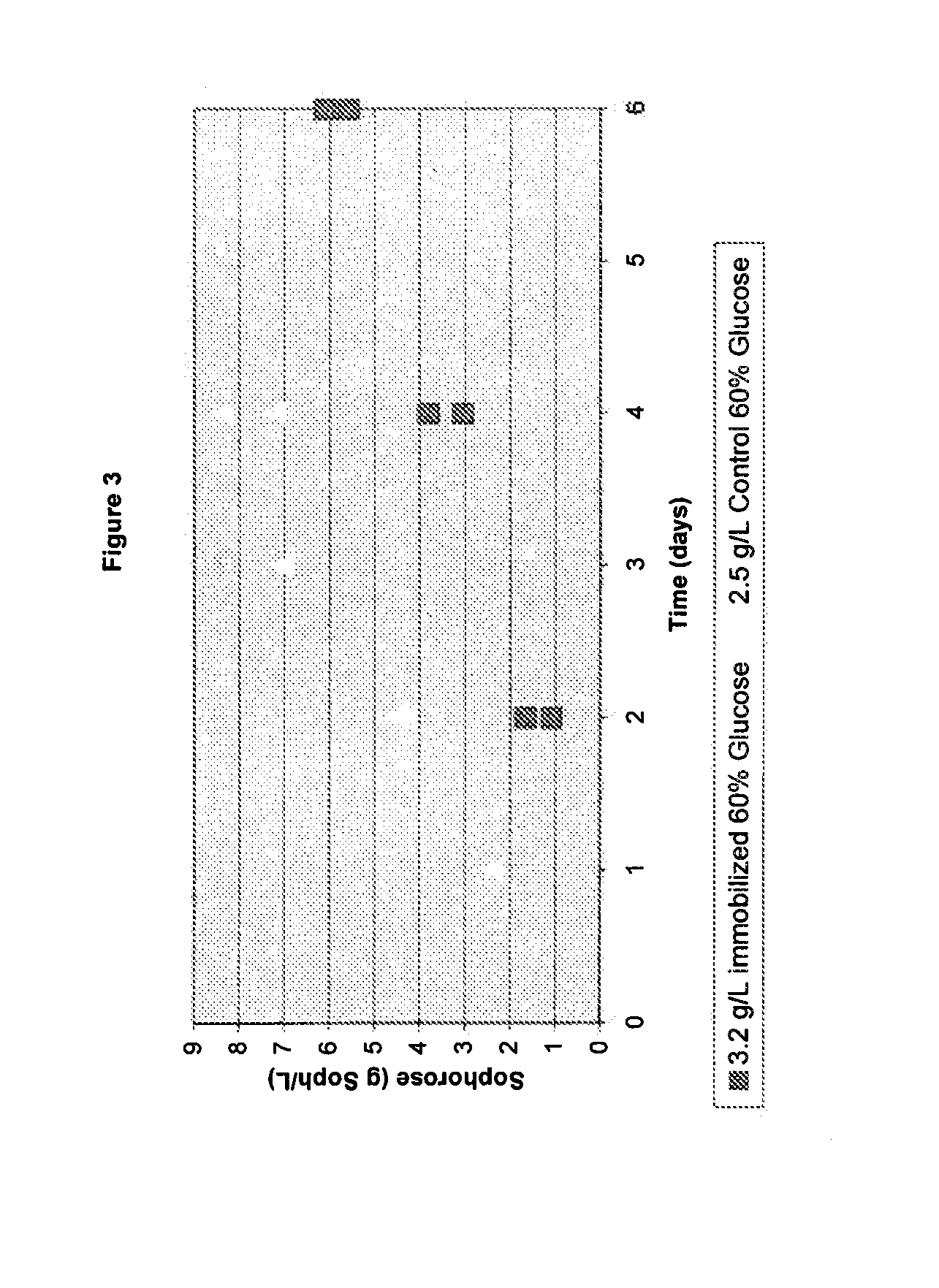
Induction of Gene Expression Using a High Concentration Sugar Mixture
a high-concentration sugar and gene expression technology, applied in the field of protein production improvement methods, can solve the problems of high cost associated with cellulase production, the problem of controlling the glucose concentration of i>trichoderma /i>grown on solid cellulose, and the problem of high cost of cellulase production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0123]This example illustrates how an inducing feed composition for stimulating the expression of cellulase genes in Trichoderma reesei was prepared. The incubation was run at the pH of the solution, i.e., 5.0. For beta-glucosidase the incubation was found to be best at pH 4.0-6.5.
[0124](i) A 60% (w / w) glucose solution was sterilized for 30 minutes at 121° C., 2.2 bar pressure.
[0125](ii) Sterile whole cellulase preparation was added to the glucose solution to a final concentration of 1 0 g total protein / L.
[0126](iii) The tank containing the glucose and whole cellulase mixture was held at 65° C. for 3 days with 75 RPM mixing.
[0127](iv) Following incubation, the sterile solution was harvested to an appropriate container for fermentation feeding.
[0128]The resulting inducing feed composition was found to have 16.1 g / L Sophorose, 47.5 g / L Gentiobiose, and approximately 600 g / L Glucose. Other sugars may be present but were not analyzed.
[0129]Inducing feed solutions have also been prepared...
example 2
[0132]The following example details how a glucose / sophorose feed is made and used to produce cellulase enzyme during fermentation.
I. Production of Glucose / Sophorose Feed:
[0133]60% (w / w) glucose solution was dissolved and sterilized for 30 minutes at 121° C. The temperature was decreased to 65° C. and 10 g of total protein (whole cellulase previously produced by T. reesei) / L was added. The mixture was agitated slowly and held at 65° C. for 3 days. The sophorose content was measured at 12 g / L in this 60% glucose solution.
II. Fermentation
[0134]0.8 L of media was inoculated with 1.5 ml Trichoderma reesei RL-P37 frozen spore suspension as a seed flask. This flask was split into two 0.4 L portions and transferred to 2×7 L of fermentation media in two different 15 L Biolafitte fermentors after 48 hours. The growth media had the following composition:
Media componentg / LKH2PO44(NH4)2SO46.35MgSO4—7H2O2CaCl2—2H2O0.53Glucose50Corn Steep Solids6.25(Roquette)Trace elements*1ml / LTrace elements*: 5 ...
example 3
[0137]The following example details how a glucose / sophorose feed is made and used to produce a heterologous protein from a filamentous fungus during fermentation.
[0138]The inducing feed composition is prepared using the procedure in Example 1.
[0139]An expression plasmid for use in transforming Trichoderma reesei is constructed as follows. The ends of the gene encoding protein of interest are blunted by T4 DNA polymerase and inserted into Pmel restriction site of the Trichoderma expression vector, pTEX, see PCT Publication No. WO 96 / 23928, which publication is herein incorporated by reference, which contains a CBH1 promoter and terminator for gene expression and a Trichoderma pyr4 gene as a selection marker for transformants. The linear DNA fragment containing only the CBH1 promoter, the gene encoding the protein of interest, the CBH1 terminator and selection marker pyr4 is isolated from a gel and used to transform a uridine auxotroph strain of Trichoderma reesei (see U.S. Pat. No. 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| w/w | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com