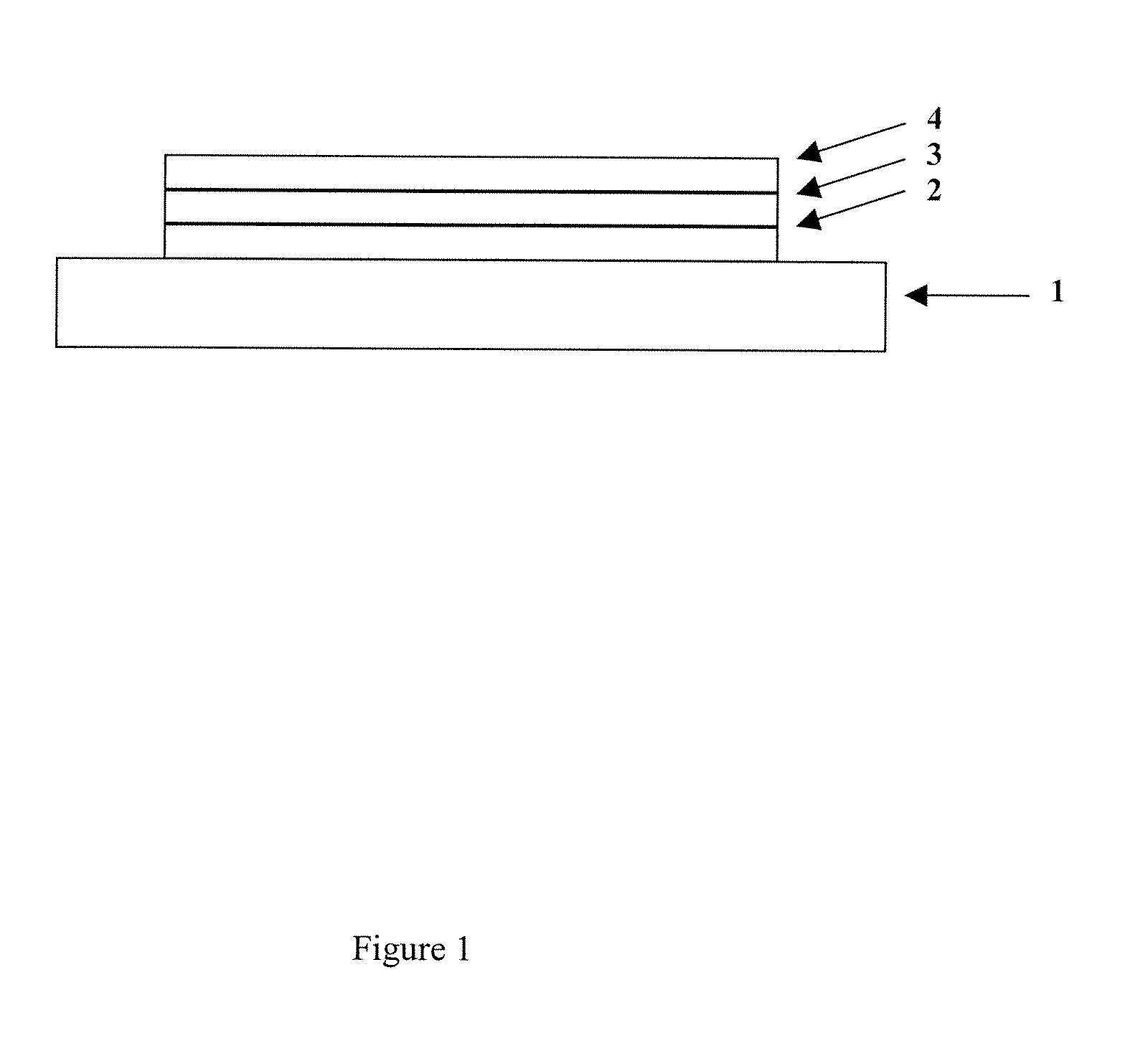
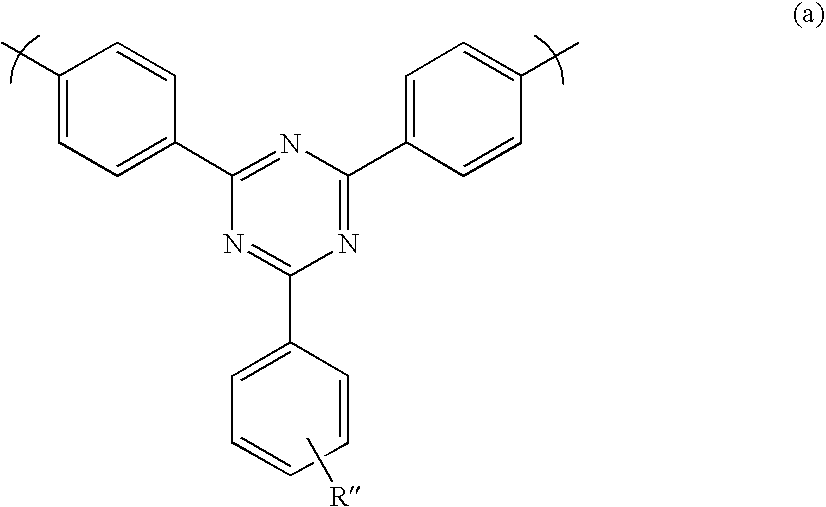
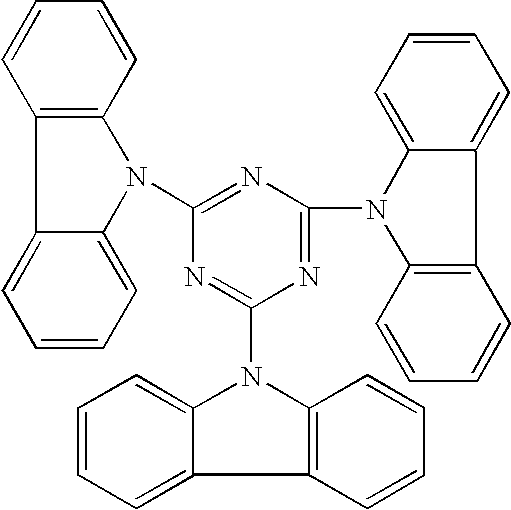
Compounds for use in Opto-Electrical Devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
2,4,6-tris(4′-bromophenyl)-1,3,5-triazine(1)
[0110]A 1 L three-necked round bottomed flask was fitted with a magnetic stirrer, a reflux condenser with N2 inlet bubbler, and a 500 ml pressure-equalising dropping funnel. The flask was charged with trifluoromethane sulfonic acid (60 g, 35 ml) that was stirred at room temperature. In a separate flask 4-bromobenzonitrile (36.4 g, 0.20 mol) was dissolved in anhydrous CHCl3 (500 ml) and the solution transferred into the dropping funnel under N2 via cannula. The benzonitrile solution was added dropwise and then the reaction mixture was heated to reflux at 90-95° C. for 20-24 hours. The reaction mixture was allowed to cool before cautiously adding to stirred dilute aqueous ammonia solution (250 ml, 3%) cooled in an ice-bath. The product precipitated as an off-white solid and was collected by filtration and washed with H2O and Et2O. The product was recrystallised from refluxing toluene to give pure product (100% by HPLC). Yield 24.0 g, 66%.
2,4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Polarity | aaaaa | aaaaa |
| Solution | aaaaa | aaaaa |
| Phosphorescence quantum yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com