Methods for the purification of polypeptide conjugates
a polypeptide and conjugate technology, applied in the field of polypeptide manufacturing, can solve problems such as unsuitable techniques
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Development of a Purification Process for the Purification of GlycoPEGylated EPO using Hydrophobic Interaction Chromatography
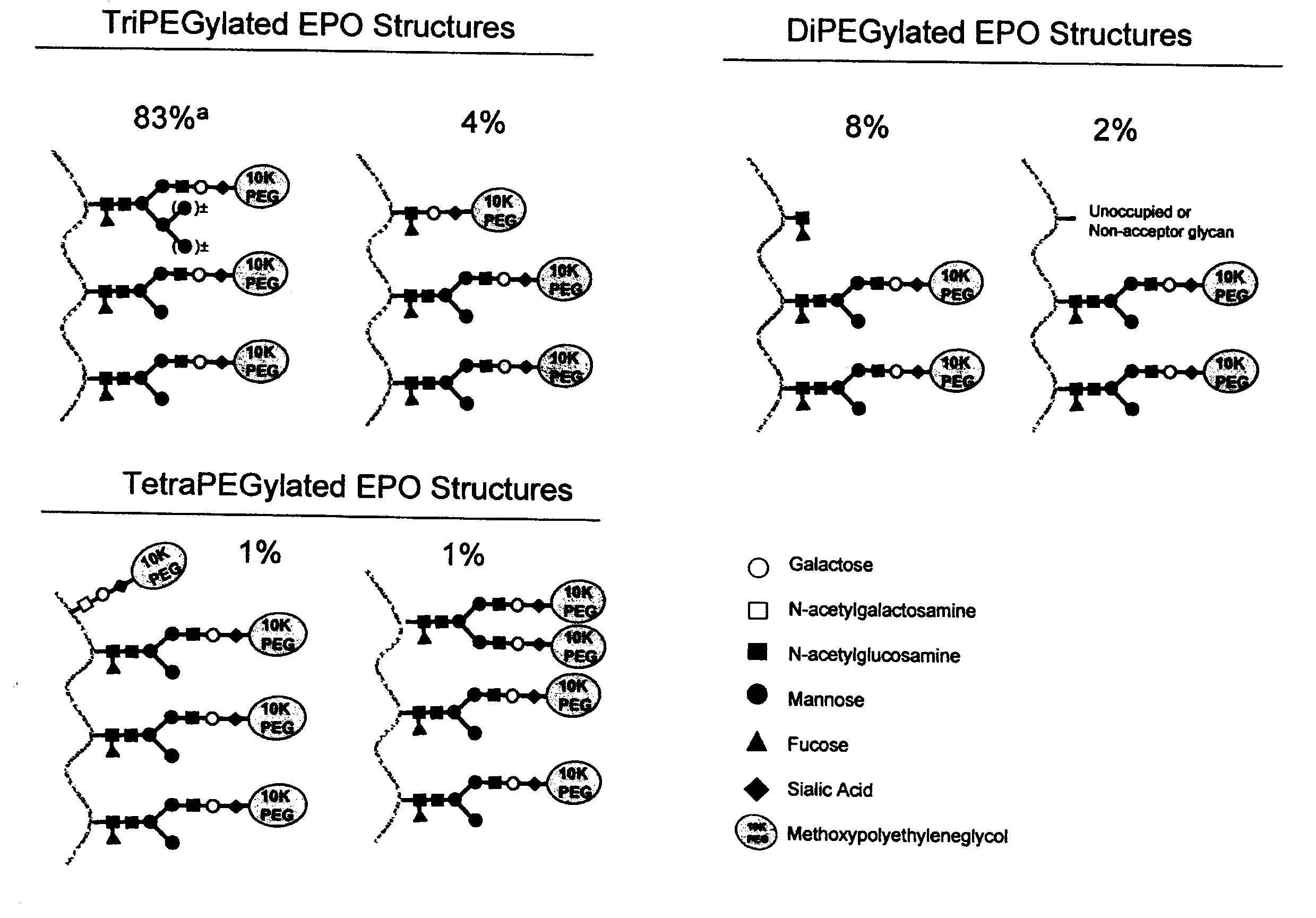
[0292]This example describes the development of an isolation process for the isolation of EPO conjugates from a glycoPEGylation reaction mixture. The resulting process is characterized by high overall EPO conjugate recovery and produces the desired EPO conjugate [EPO-(SA-PEG-10 kDa)3] in high purity. The composition produced by the process is essentially free of other EPO-PEG glycoforms, such as mono-, di- and tetra-PEGylated EPO conjugates.
[0293]The desired EPO-PEG conjugate is a glycoPEGylated erythropoietin protein that contains three 10 kDa mPEG groups attached to each of the three monoantennary N-linked glycans. The EPO polypeptide is produced by expression of the protein from Sf9 cells using a Baculovirus infection protocol. The insect cell expression system produces EPO with three N-linked glycans, at Asn24, Asn38 and Asn83, each containing a trimannosy...
example 2
Large-Scale Purification of GlycoPEGylated EPO using Hydrophobic Interaction Chromatography
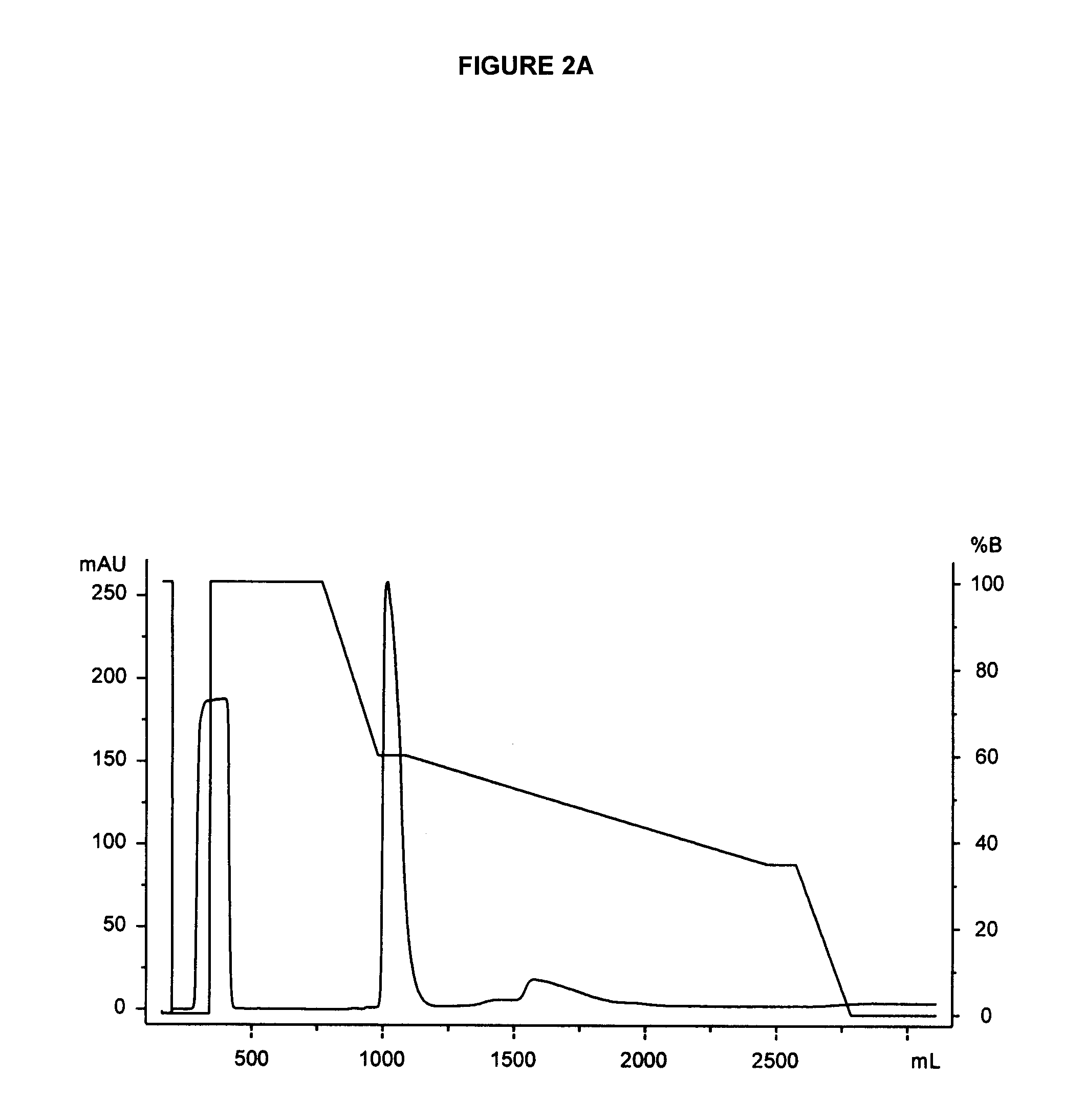
[0330]In order to further purify glycoPEGylated EPO from a glycoPEGylation reaction mixture, and to separate the trifunctionalized EPO species [EPO-(SA-PEG-10 kDa)3] from other PEGylated species (e.g., mono-, di- and tetra-PEGylated EPO species), the mixture (e.g., flowthrough from an anion chromatography medium) is subjected to hydrophobic interaction chromatography (HIC). An exemplary HIC procedure is outlined below: An XK26 column was packed with Phenyl 650S resin (106 mL, 2.6 cm×20 cm) and attached to an AKTA Explorer 100 system. Product elution was monitored by absorbance at 214, 254 and 280 nm. The column was equilibrated with 5 column volumes (CV) of Buffer B (25 mM sodium phosphate, 0.6 M sodium sulfate, pH 7.5)
[0331]The solution of Sartobind Q purified EPO-(SA-PEG-10 kDa)1-4 (62 mL, pH 6.94, 2.59 mS / cm) was adjusted to 0.6 M sodium sulfate by dilution with 62 mL of 1.2 M of buffer (1....
example 3
Preparation and Isolation of EPO-(SA-PEG-10 kDa)3 from a GlycoPEGylation Reaction Mixture
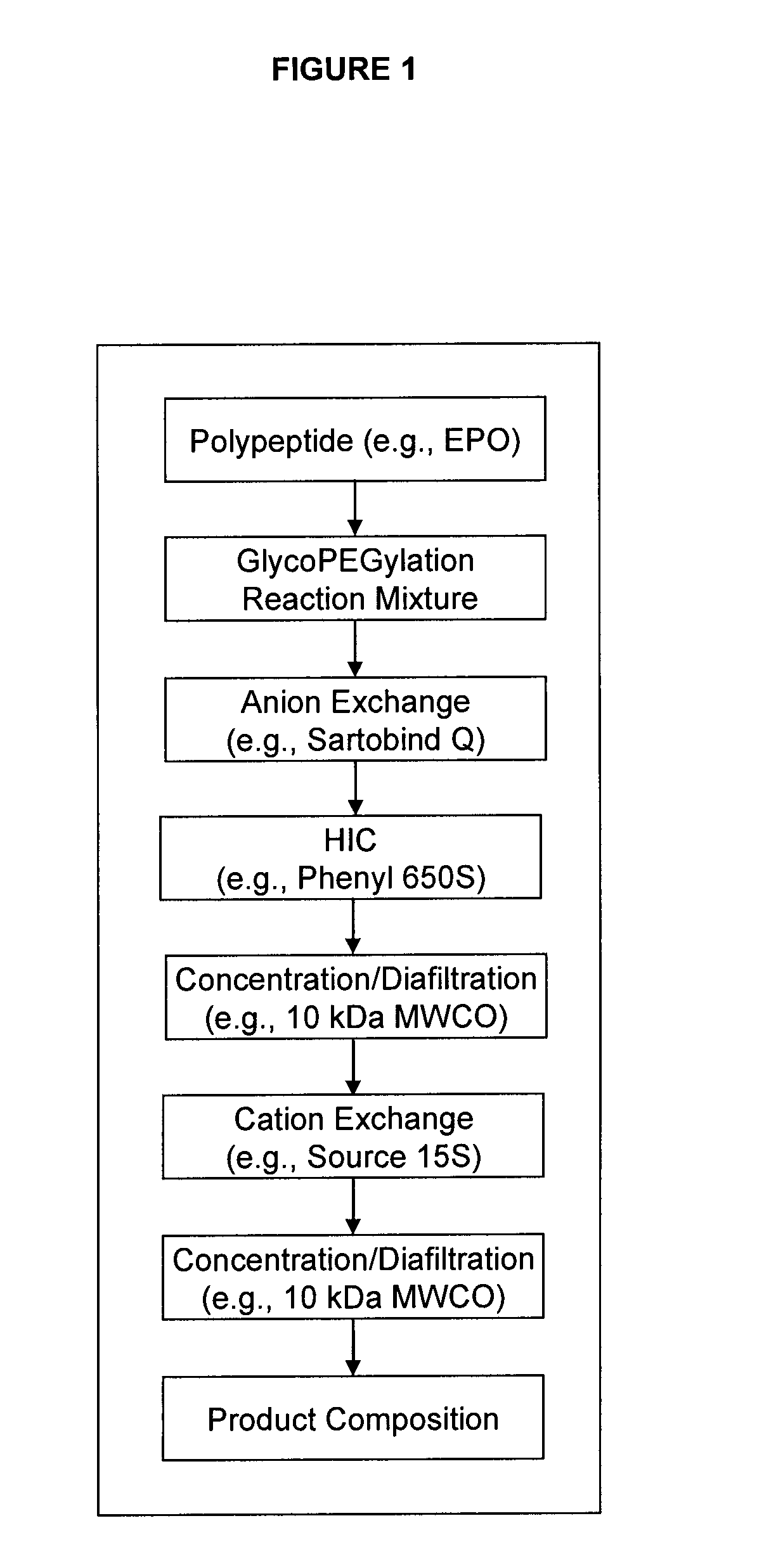
[0334]This example summarizes the results obtained with a 3 chromatography step (and two ultrafiltration / diafilatration (UF / DF) steps) purification process for the isolation of baculoviral derived, glycoPEGylated human erythropoietin EPO-(SA-PEG-10 kDa)3 (see, description of EPO-PEG conjugates in Example 1, above) at a 20 mg scale. Overall process efficiency and product quality were assessed.
[0335]The purification process (see e.g., FIG. 1) began with a Sartobind Q membrane used in a negative binding mode which allowed the PEG-EPO conjugates to flow through while capturing glycoPEGylation enzymes, such as MBP-GnT 1, MBP-GalT 1, MBP-SBD-ST3Gal3 and other enzyme contaminants. The various PEG species generated in the glycoPEGylation reaction were then fractionated using HIC on a Phenyl 650S resin (also compare Example 1), which enriched the EPO-(SA-PEG-10 kDa)3 to a concentration of >96% (step yiel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com