Methods For Producing Sodium Hypochlorite With a Three-Compartment Apparatus Containing an Acidic Anolyte
a technology of sodium hypochlorite and acidic anolyte, which is applied in the direction of electrolysis process, electrolysis components, cells, etc., can solve the problems of inefficient and expensive methods for producing sodium hypochlorite, and require relatively large amounts of electricity to be spent, so as to increase the longevity of electrolytic cell operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
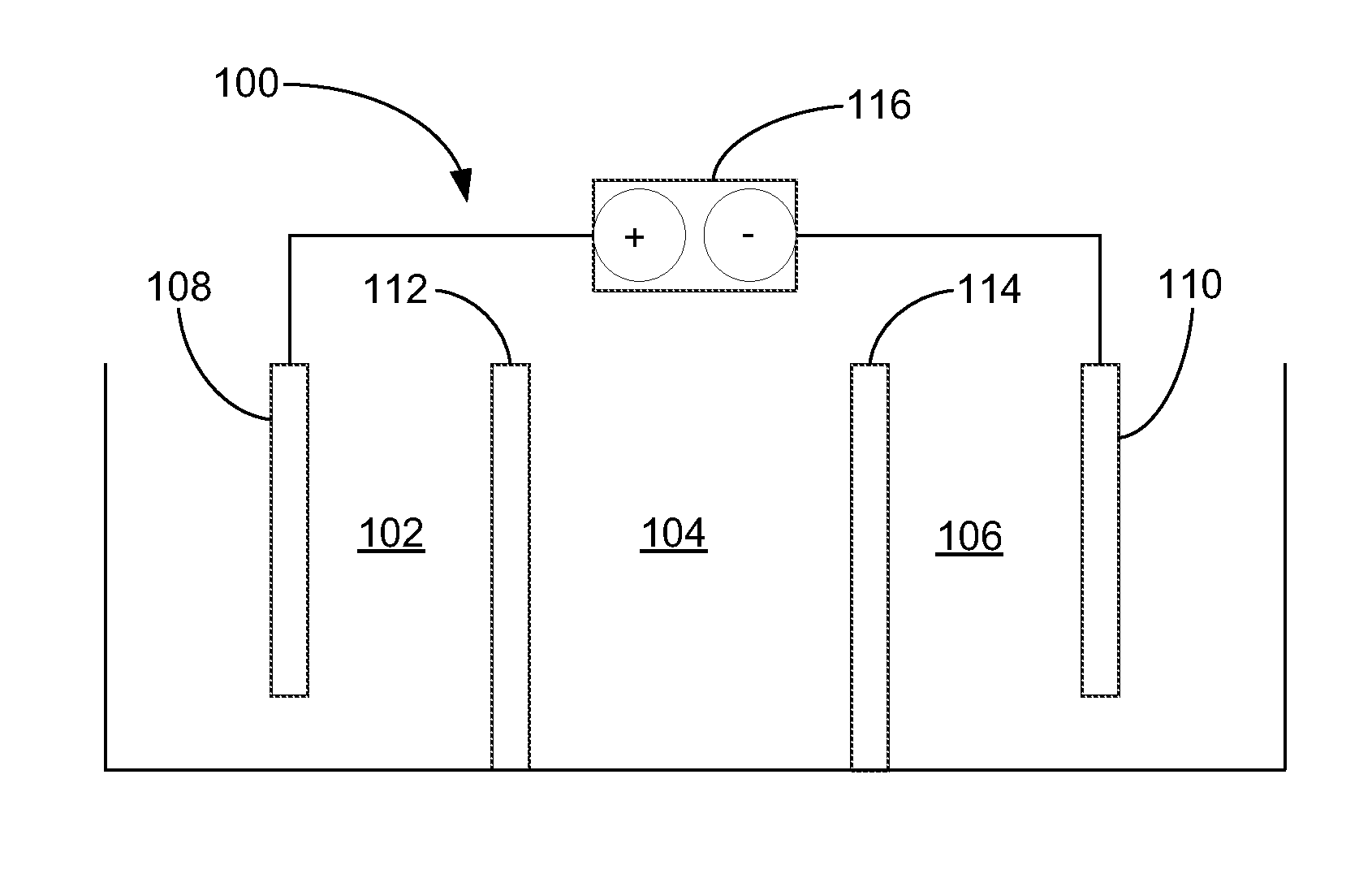
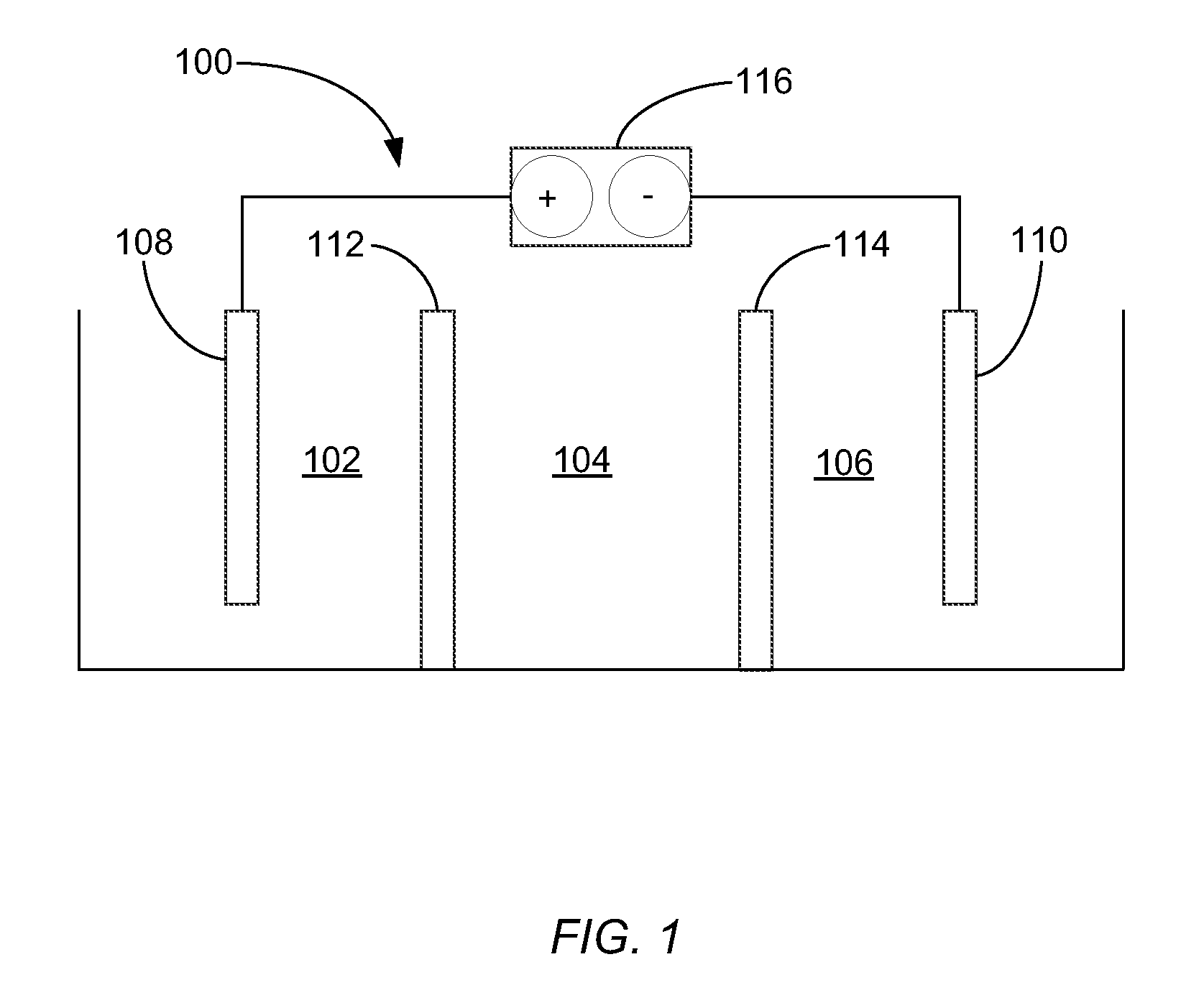
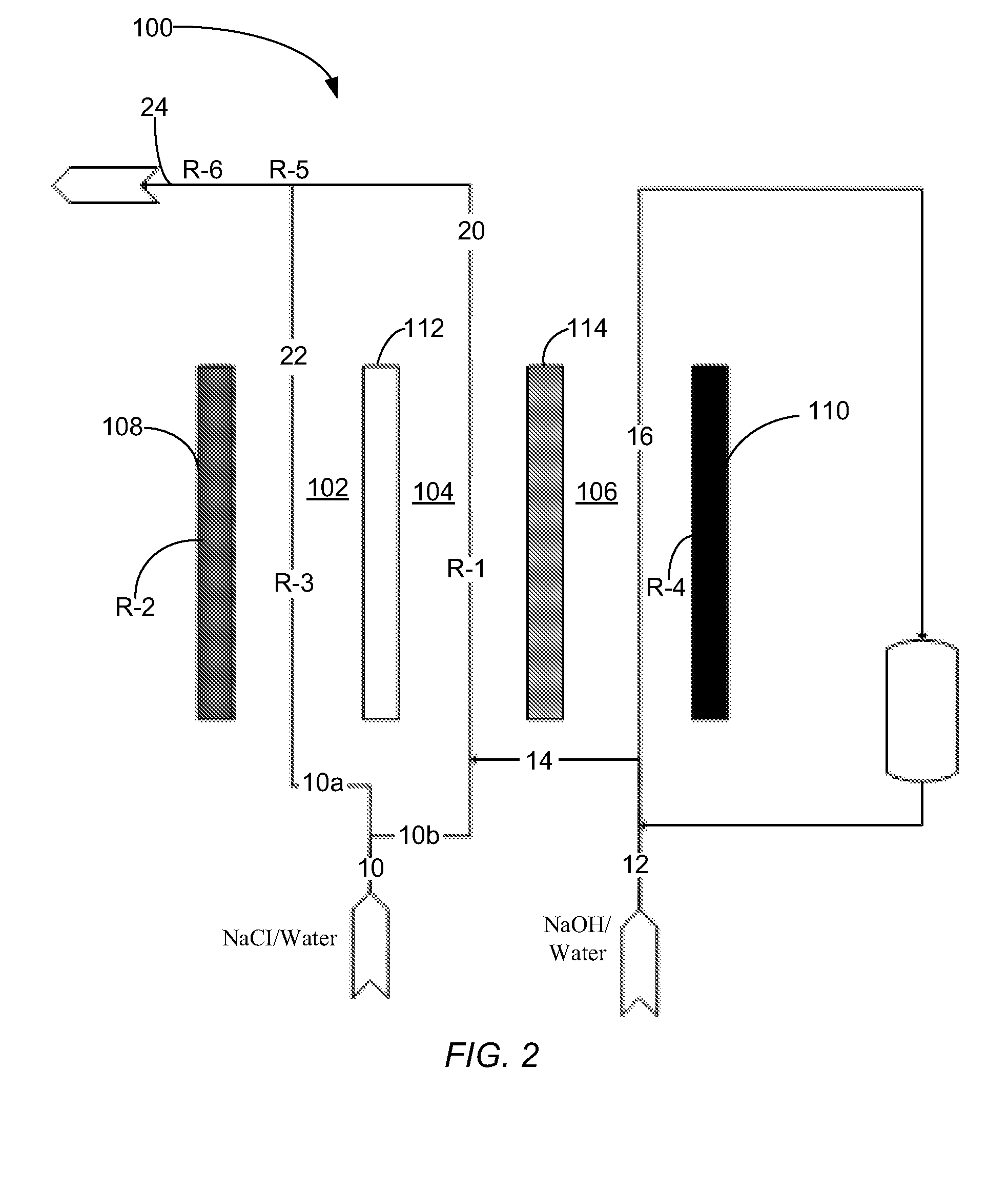
[0081]In one example of how the electrolytic cell functions, a cell was prepared and operated according to the systems and methods shown in FIG. 3 (Scheme B). Specifically, the cell was prepared to include an ACS anionic membrane and a NaSICON ceramic cationic membrane. A catholyte comprising 10 wt % sodium hydroxide was introduced to, and continuously re-circulated through, the third compartment. Moreover, an electrolyte comprising about 10 wt % sodium chloride was introduced to, and continuously re-circulated through, the second compartment. Additionally, an anolyte comprising tap water and about 3.5 wt % sodium chloride was introduced into the first compartment. With the described fluids in each compartment, the cell was operated at a current density of about 40 mA / cm2. An effluent from the first compartment was collected at a flow rate of about 0.25 L / h (e.g., about 4.2 ml / min) and was found to comprise the hypochlorite ion at a concentration of 4.23 g / L. The power consumption f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wt % | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com