Melatonin tablet and methods of preparation and use
a technology of melatonin and tablets, which is applied in the field of melatonin tablets and methods of preparation and use, can solve the problems of poor oral bioavailability, poor and erratic melatonin bioavailability, and inability to provide rapid onset of action, so as to improve oral mucosal absorption, enhance drug action, and reduce the effect of toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
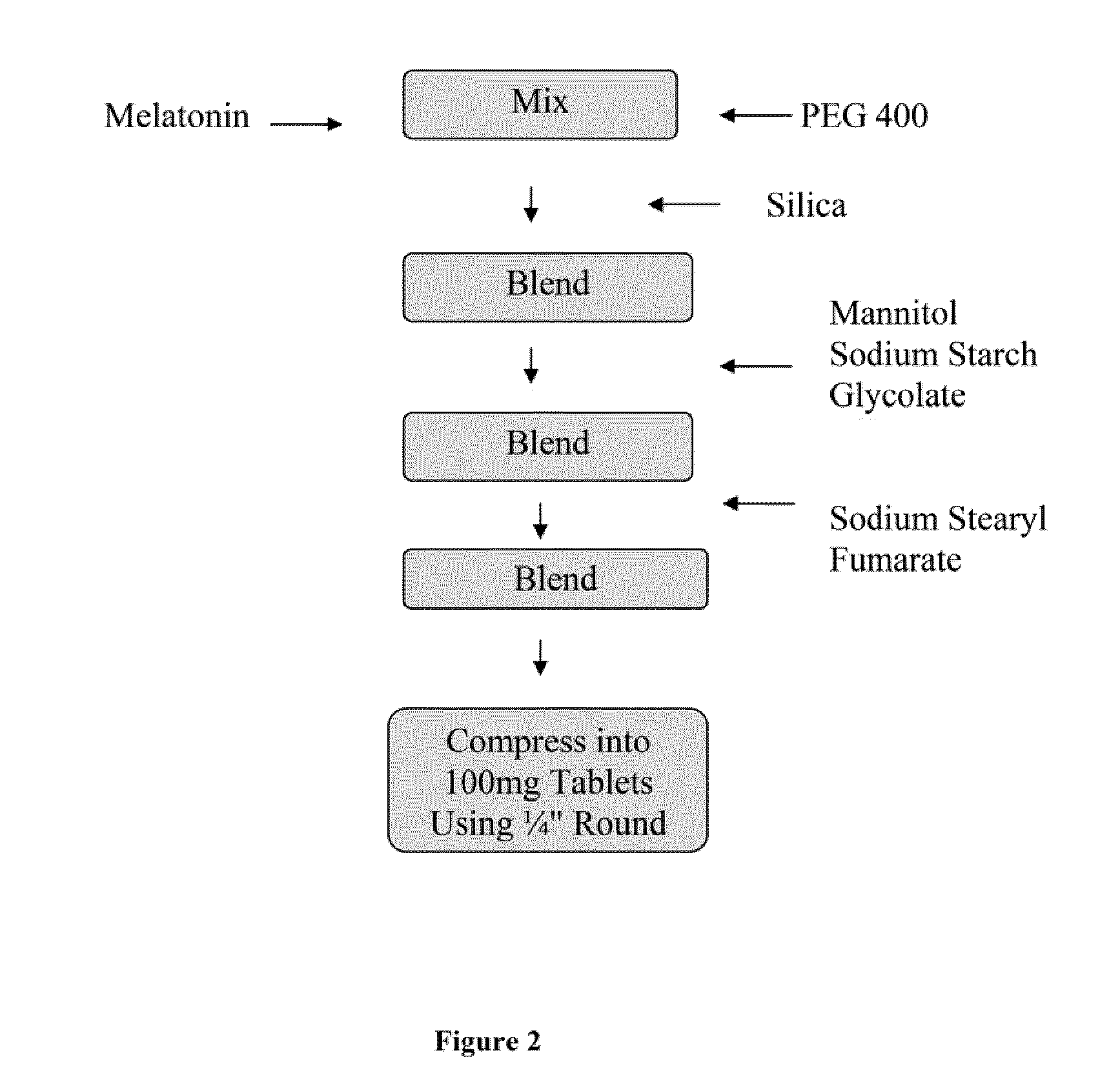
[0035]In one embodiment, the invention provides a 1 mg strength melatonin sublingual / buccal tablet having a total tablet weight of about 100 mg, wherein the tablet comprises drug, an absorbent / adsorbent particulate carrier, such as silica; a diluent, such as mannitol; a disintegrent, such as sodium starch glycolate; and a lubricant, such as sodium stearyl fumarate, to facilitate tableting. In such an embodiment, melatonin is dissolved in PEG 400. The melatonin-in-PEG 400 solution is then processed into a into a flowable powder suitable for use in direct compression tableting. An exemplary formulation in accordance with the described formulation of this embodiment is provided in Table I, below.
TABLE I1 mg Melatonin Sublingual / Buccal Tablet FormulationINGREDIENTAMOUNT (mg / tablet)Melatonin1.00Polyethylene glycol 4007.00Silica4.50Mannitol84.00Sodium Starch Glycolate3.00Sodium Stearyl Fumarate0.50Total Tablet Weight100.00
example 2
[0036]In one embodiment, the invention provided a 1 mg strength melatonin sublingual / buccal tablet having a total tablet weight of about 68 mg. In this second exemplary embodiment, melatonin is dissolved in a mixture of solvents, PEG 400 and oleic acid. In order to convert the melatonin PEG 400 / Oleic Acid solution into a flowable powder suitable for use in direct compression tableting, an adsorbent / absorbent particulate carrier, such as silica, can be used as above in Example 1. A tablet diluent, such as mannitol can be used for formulating a directly compressible tablet. Sodium starch glycolate was used as a disintegrant, and sodium stearyl fumarate was usd as a lubricant.
[0037]An exemplary formulation manufactured for this embodiment in accordance with the subject invention are provided in Table II, below.
TABLE II1 mg Melatonin Sublingual / Buccal Tablet FormulationINGREDIENTAMOUNT (mg / tablet)Melatonin1.00Polyethylene glycol 4005.00Oleic Acid1.30Silica5.00Mannitol52.30Sodium Starch ...
example 3
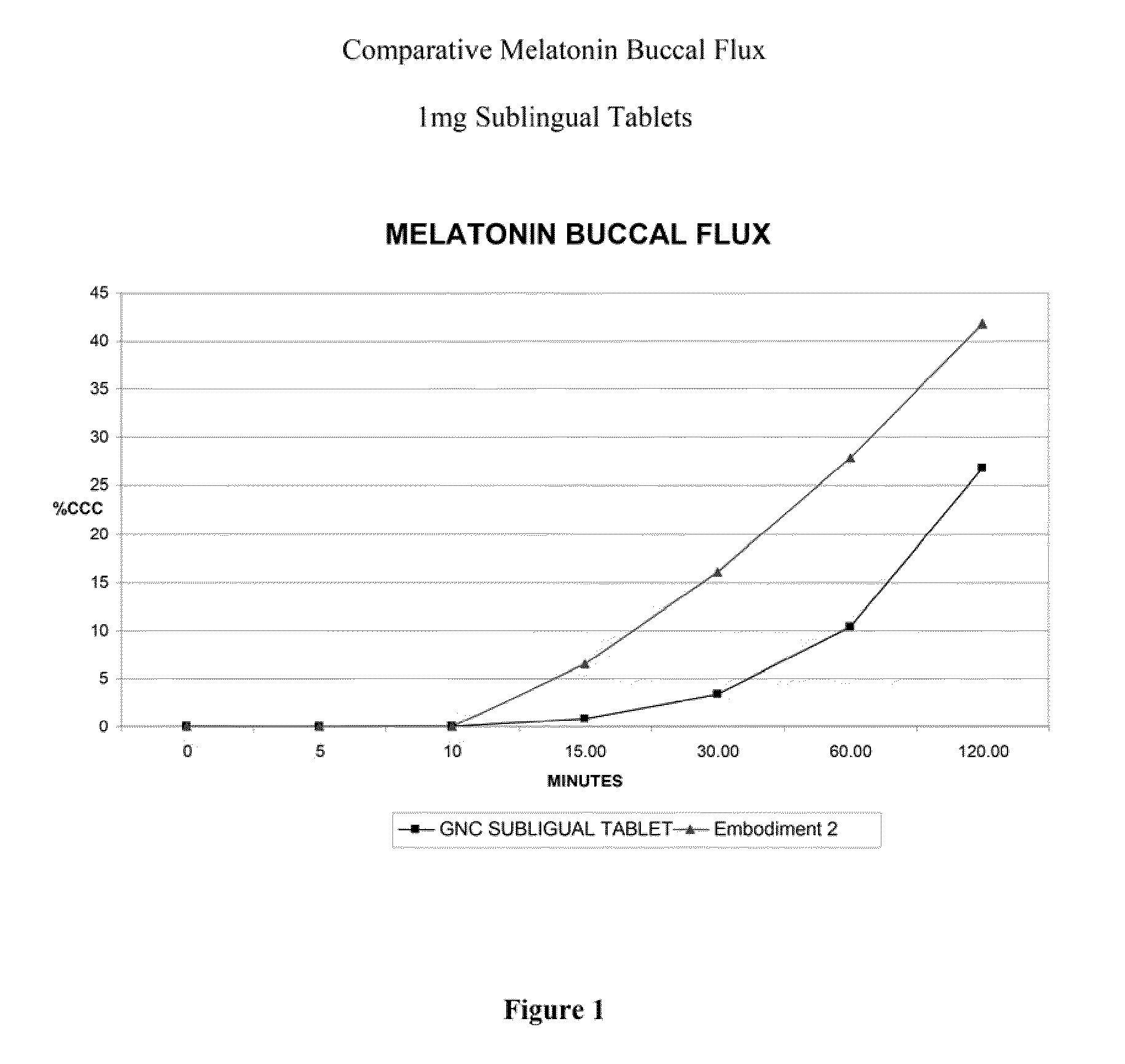
[0038]An in vitro buccal skin flux study was conducted comparing melatonin permeation through buccal tissue culture from two 1 mg sublingual melatonin tablets having a formulation according to Example 2, against a commercially available sublingual tablet which does not include dissolved melatonin in the final dosage form. As shown in FIG. 1, the amount of melatonin that permeated the tissue was more than 3-fold greater after 30 minutes from a tablet of the subject formulation compared to a commercial GNC 1 mg melatonin sublingual tablet, as measured as percent label concentration (% LC). This shows enhanced rate of buccal tissue permeation of the invention as compared to a currently marketed sublingual melatonin tablet, which suggests a faster onset of action and greater bioavailability for the subject tablets in vivo. Therefore, it may be concluded that the onset of sleepiness would be much faster using a formulation in accordance with the subject invention, such as the formulation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com