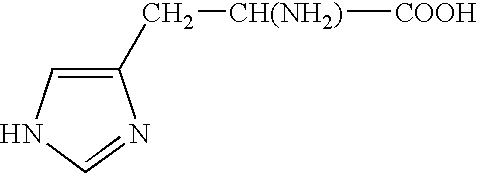Peptide nanoparticles and uses therefor
a technology of nanoparticles and peptides, which is applied in the field of peptide nanoparticles and uses therefor, can solve the problems of less effective, less effective, and less effective biologically, and achieve the effect of preserving the biological activity of the peptide, reducing the cost of production of inventive cosmetic and/or pharmaceutical preparations, and simplifying and reducing the cost of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Pentapeptide Nanoparticle Formulation
[0143]This example presents peptide nanoparticle compositions comprising a nanoemulsion containing a pentapeptide, KTTKS (SEQ ID NO.: 1), that is known to have biological activity on the skin structures (Katayama et al., supra).
[0144]A pentapeptide nanoemulsion preparation was prepared as follows:[0145]800 mg of soybean oil and 800 mg of Tween 80 were stirred in a sterile vial for 5 minutes;[0146]8.4 ml water with 0.0001 g of the peptide KTTTS (SEQ ID NO.: 1) was added and stirred for 20 minutes;[0147]The sample was homogenized for 1 minute; The sample was stirred for 20 minutes; and[0148]The sample was microfluidized once at 23,000 psi.
[0149]The resulting pentapeptide nanoemulsion was evaluated for particle size using the Malvern Nano S particle sizer capable of sizing particles between about 0.6 nm 6000 nm. The pentapeptide nanoemulsion preparation had two particle size peaks having an average particle size of 106 nm (Table 2).
TABLE 2Particle S...
example 2
Pentapeptide Nanoparticle Formulation and Transdermal Penetration with Biological Effect
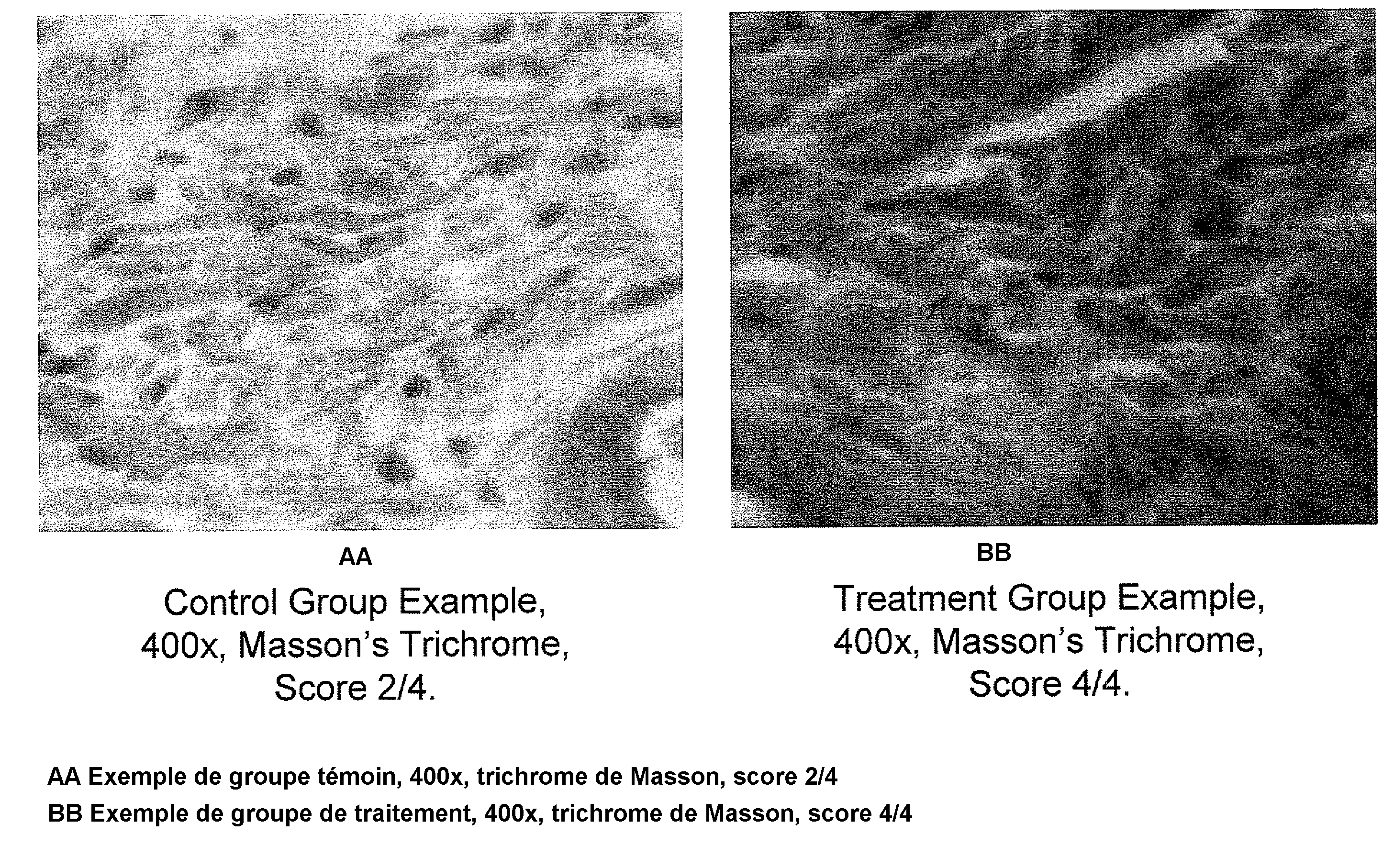
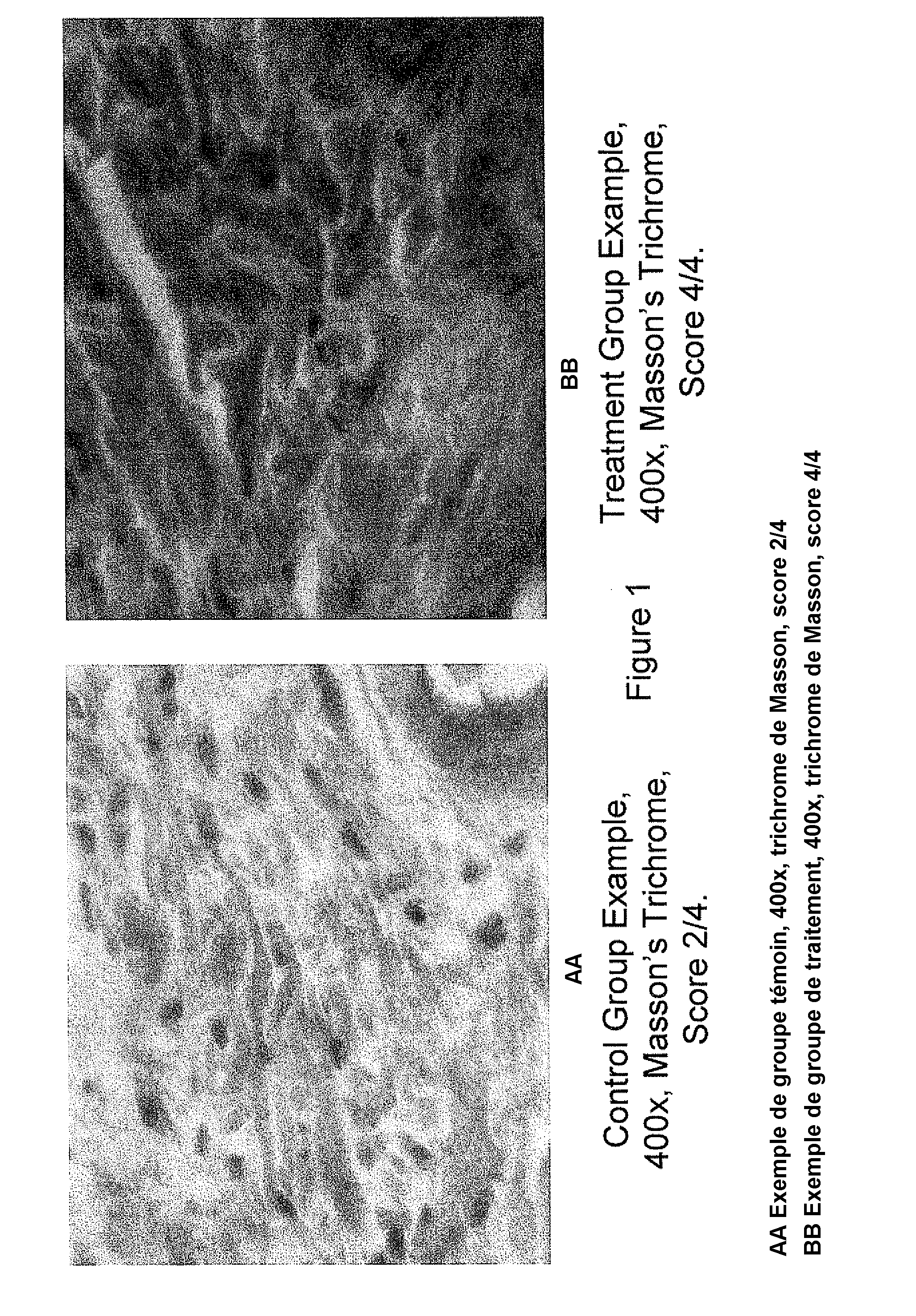
[0150]This example presents peptide nanoparticles comprising a nanoemulsion containing a pentapeptide, KTTKS (SEQ ID NO.: 1), that is known to have biological activity on the skin structures (Katayama et al., supra; incorporated herein by reference). This example demonstrates the biological efficacy on the skin of transdermally applying a peptide nanoparticle, in this case KTTKS (SEQ ID NO.: 1).
Materials and Methods
[0151]A pentapeptide nanoemulsion preparation was prepared as follows:[0152]5.6 g of Labrafac WL 1349 oil and 5.6 g of Tween 80 were stirred in a sterile beaker for 5 minutes;[0153]58.8 g Reagent Grade water was placed in a separate beaker; 0.010 g of the peptide KTTTS (SEQ ID NO.: 1) was added into the water and stirred for 20 minutes;[0154]The contents of the first beaker were added to the contents of the beaker (i.e., the water and peptide) and then stirred for 20 minutes; and[0155]...
example 3
Skin Thickening and Extra-Cellular-Matrix Stimulator Effects on Mice Through Transdermal Application of a Peptide Nanoparticle: Effect of Varying Concentration of Peptide in the Nanoparticle
[0174]This example demonstrates the impact of varying the concentration of peptide in the nanoparticle on the biological efficacy on the skin of transdermally applying a peptide nanoparticle.
Materials and Methods
[0175]The experiment described Example 3 is repeated, except that the concentration of peptide in the Treatment cream is decreased by a factor of ten or increased by a factor of ten.
Results and Conclusion
[0176]The results are expected to show that, on average, those mice treated with the peptide concentration increased by a factor of ten have statistically thicker skin those mice treated with the increased peptide concentration. The results are expected to show that, on average, those mice treated with the peptide concentration increased by a factor of ten have statistically more collagen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameters | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com