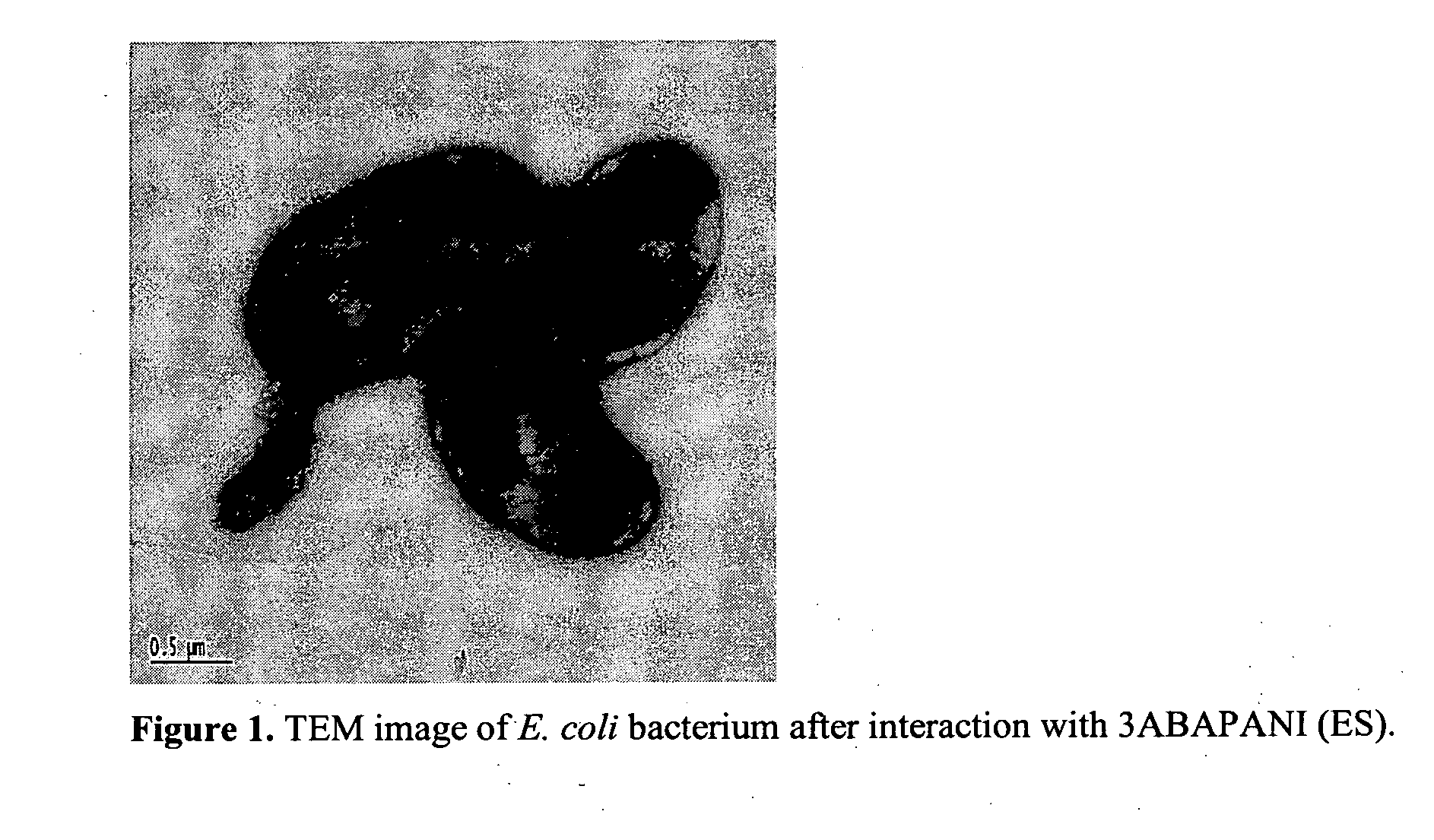
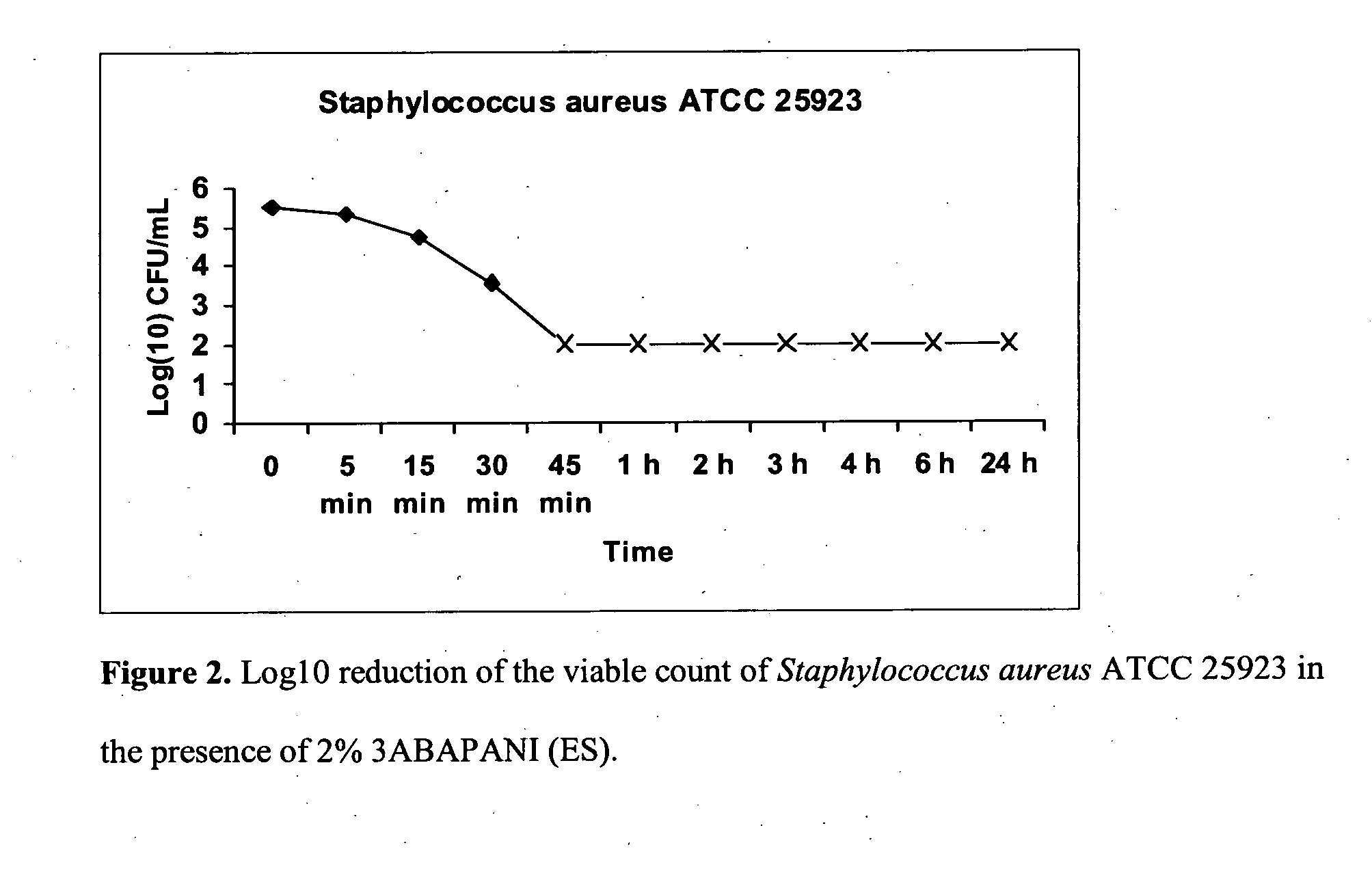
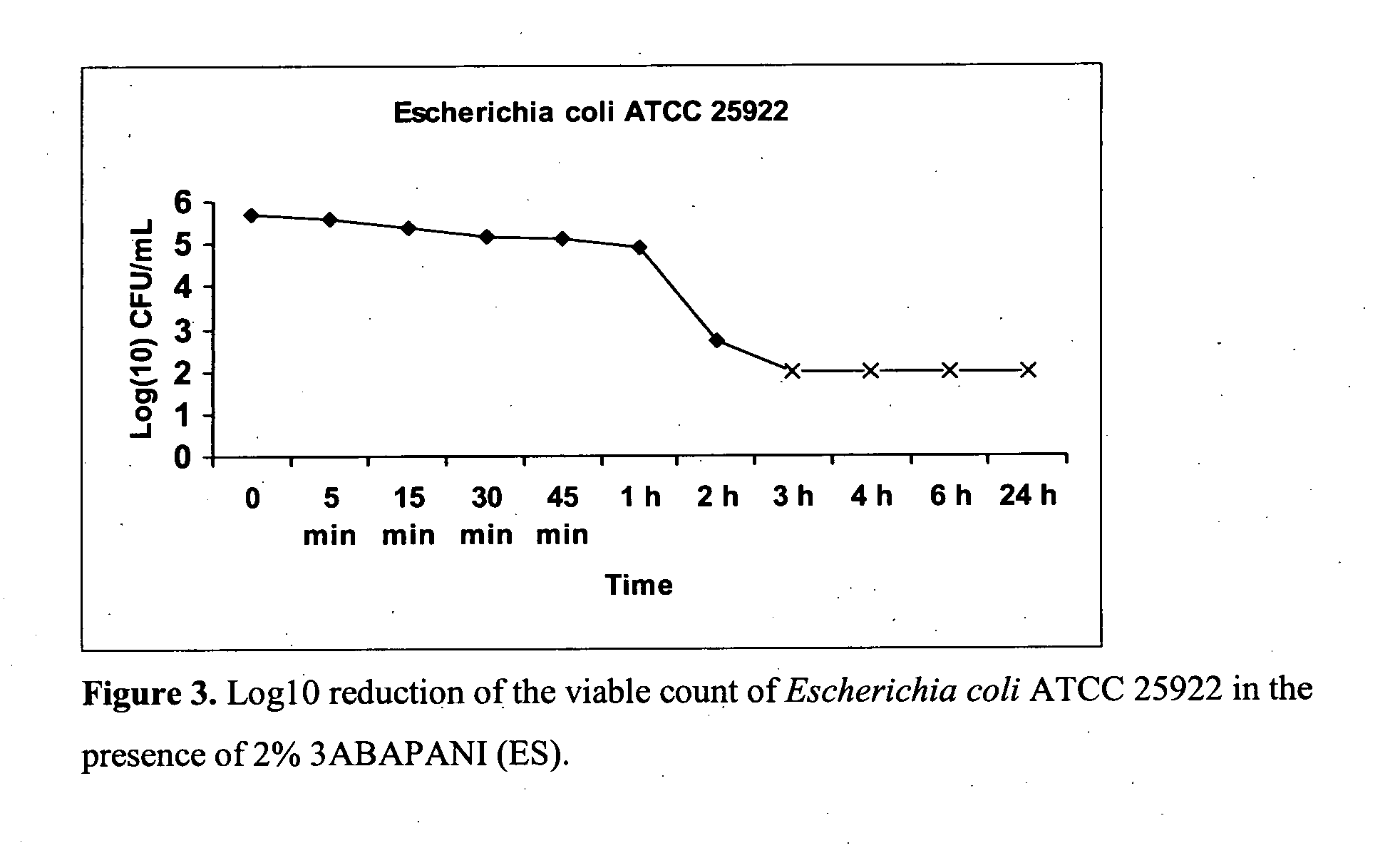
Bioactive aniline copolymers
a technology of aniline copolymer and bioactive aniline, which is applied in the field of polyaniline copolymer, can solve the problems of poor processing ability, inability to widely exploit polyaniline in technological applications, and significant cost disadvantages of hfp use, and achieves easy incorporation into films or gels, fast inhibitory effect, and easy processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Synthesis of Copolymers
[0184]The 1:1 copolymer synthesis of aniline with 3-amino benzoic acid (3ABAPANI) or aniline with anthranilic acid (OABAPANI) was performed using 3.88 mL aniline, 5.85 g 3-amino benzoic acid or anthranilic acid respectively, 8.64 g of potassium iodate (KIO3) and 240 mL of 1.25 M hydrochloric acid.
[0185]The 1:1 copolymer synthesis of aniline with ethyl 3-amino benzoate (3EABPANI) was carried out using 0.9 mL of aniline, 1.65 g of ethyl 3-aminobenzoate, 62.5 mL of 1.25 M HCl and 2.25 g of KIO3.
[0186]After cooling the solution of potassium iodate and hydrochloric acid at 7° C., aniline and functionalised aniline monomers were used in 1:1 mole ratio. The solution was stirred for 5 hours at 7° C. to obtain emeraldine salt (ES) form. The reaction mixture was filtered and washed thoroughly with distilled water and the filtrate was transferred to a flask and stirred overnight with 150 mL (or 46.8 mL in the case of 3EABPANI) of 6% ammonia solution to dedope the polymer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Structure | aaaaa | aaaaa |
| Ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com