Nutraceutical co-crystal compositions
a technology of co-crystals and compositions, which is applied in the field of co-crystal compositions containing, can solve the problems of relatively unexplored co-crystals and the acquisition of non-uniform mixtures, and achieve the effects of improving animal health or nutrition, improving properties, and increasing aqueous solubility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Methanol Solvate of Co-Crystal of Quercetin and Caffeine—KP05
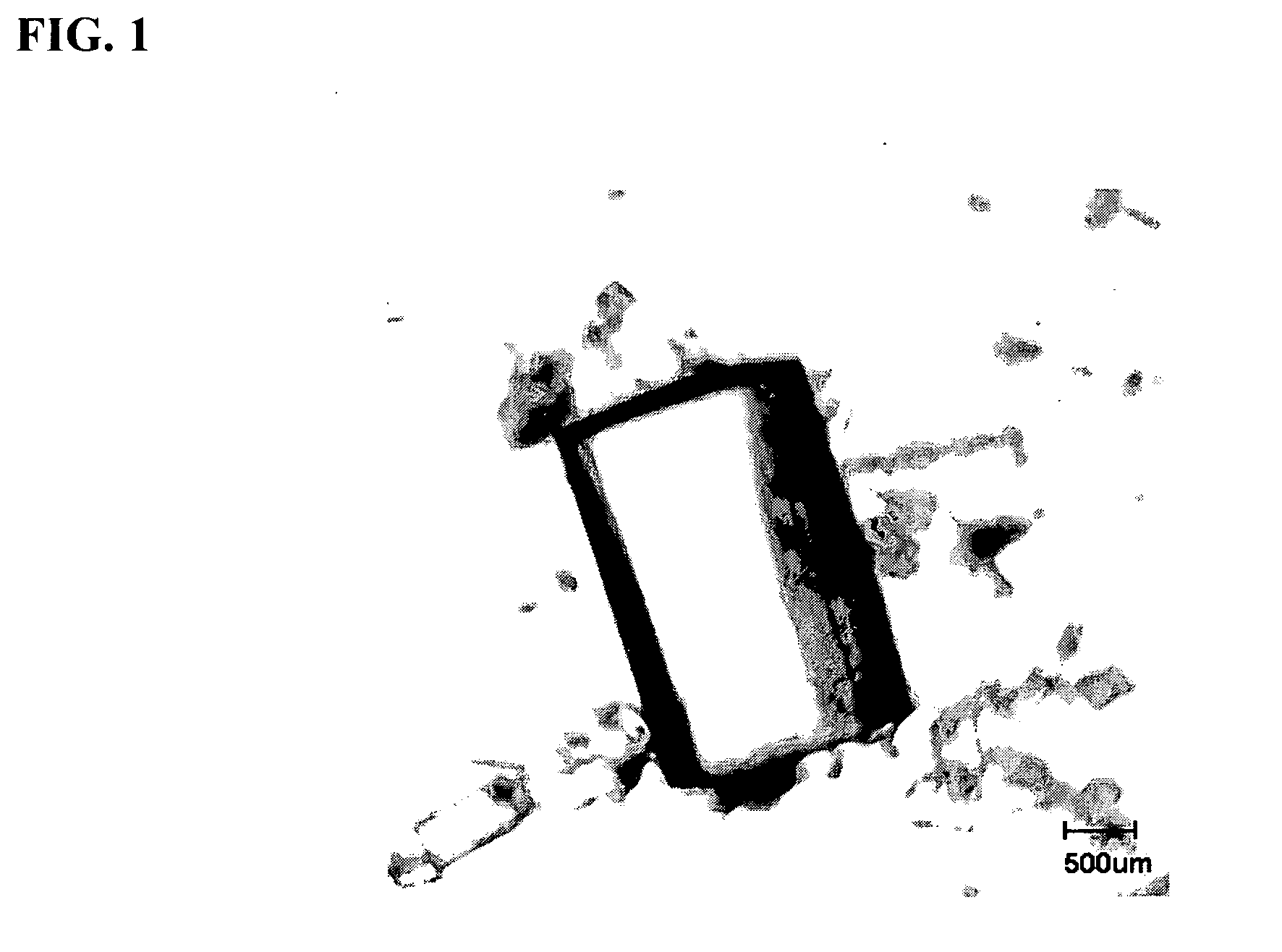
[0174]34.0 mg (0.101 mmol) of quercetin dehydrate (98% pure, Sigma Aldrich) and 19 mg (0.100 mmol) of caffeine (Sigma Aldrich) were dissolved in approximately 6 mL methanol by heating. Slow evaporation of the solvent yielded golden yellow crystals of quercetin:caffeine (1:1) co-crystal (hereinafter, “KP05”). A digital microscopic image of KP05 is shown in FIG. 1 of the accompanying drawings. The reaction scheme is shown below:
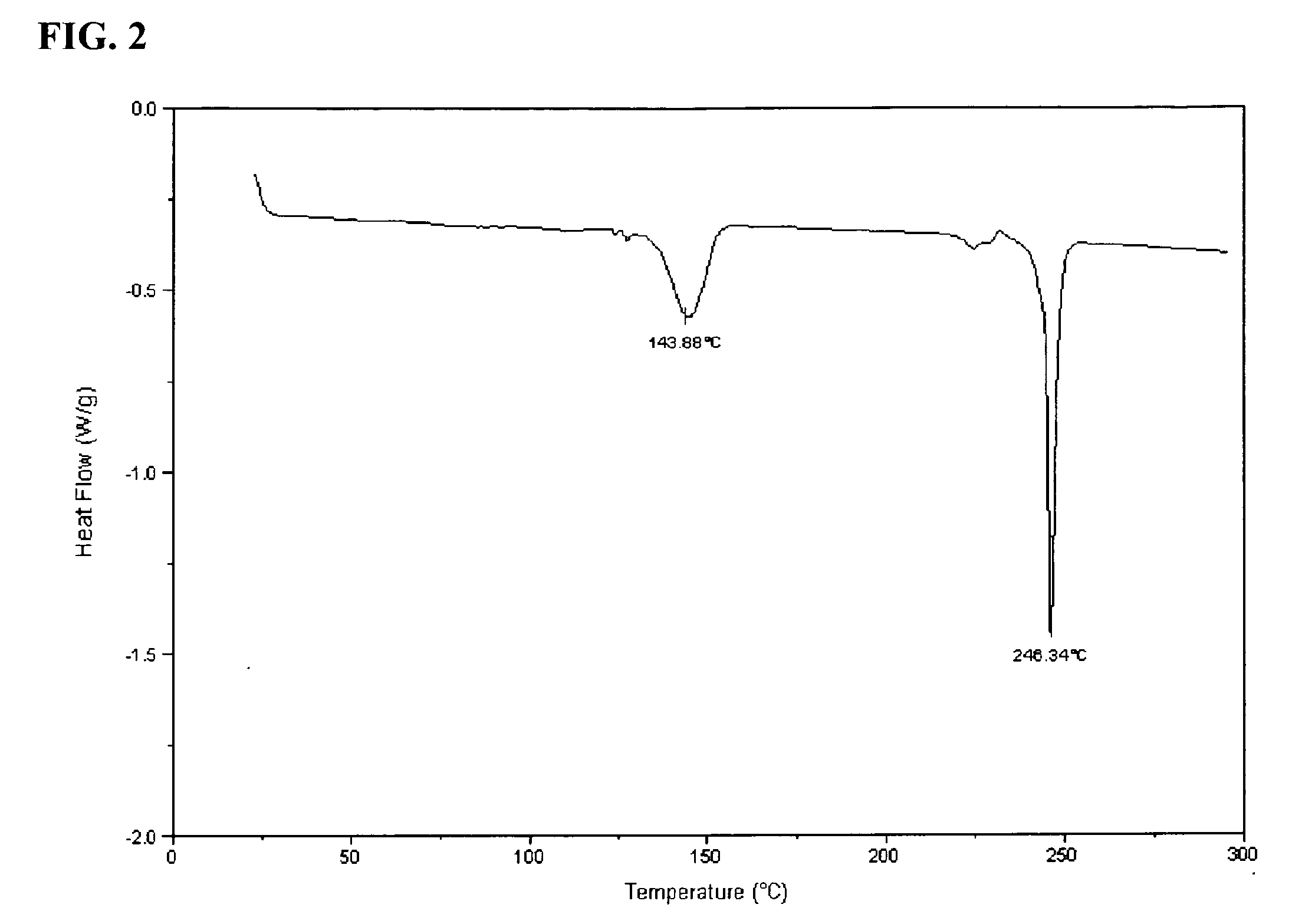
[0175]KP05 was characterized by DSC analysis, which showed phase changes at about 144° C. and about 246° C. The DSC graph is shown in FIG. 2 of the accompanying drawings.
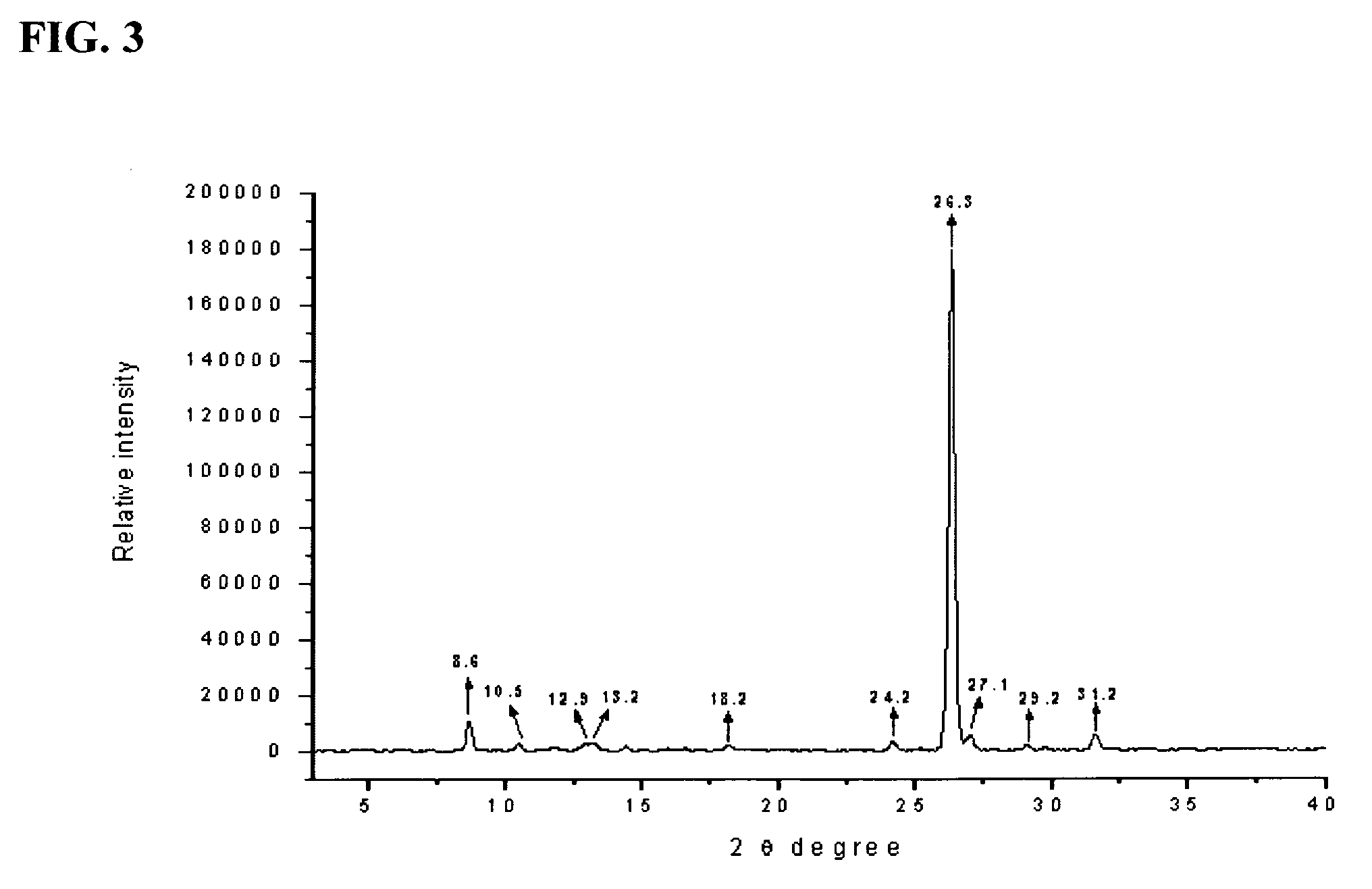
[0176]KP05 can be characterized using XRPD, and exhibited major peaks at about the following positions: 8.6, 10.5, 18.2, 24.2, 26.3, 29.2, and 31.2 degrees. The experimental XRPD graph for KP05 is shown in FIG. 3 of the accompanying drawings. The calculated XRPD graph for KP05 is shown in FIG. 4 of the accompanying drawings, and a com...
example 2
Co-Crystal of Quercetin and Iso-Nicotinamide—KP10
[0181]34.0 mg (0.101 mmole) of quercetin dehydrate (98% pure, Sigma Adrich) and 12.3 mg (0.100 mmol) iso-nicotinamide (Sigma Aldrich) were dissolved in approximately 6 mL of methanol by heating. The resulting solution was cooled in a refrigerator and allowed to stand for two days. Slow evaporation yielded golden yellow crystals of 1:1 quercetin:iso-nicotinamide co-crystal (hereinafter “KP10”). A digital microscopic image of KP10 is shown in FIG. 10 of the accompanying drawings. KP10 can also be prepared by slurrying in methanol overnight. The reaction scheme is shown below:
[0182]DSC analysis was performed on KP10 and showed a phase change at about 262° C. The DSC graph is shown in FIG. 11 of the accompanying drawings.
[0183]KP10 can be characterized using XRPD, and exhibited major peaks at about the following positions: 6.2, 7.7, 14.1, 15.4, 21.5, 24.0, 26.5, 27.9, and 30.9 degrees. The experimental XRPD graph for KP10 is shown in FIG....
example 3
Co-Crystal of Hesperetin and Iso-Nicotinamide—KP15
[0188]30.2 mg (0.0100 mmol) hesperetin (95% pure, Sigma Aldrich) and 12.2 Mg (0.0100 mmol) iso-nicotinamide (99% pure, Sigma Aldrich) were dissolved in approximately 8 mL of methanol by heating. The resulting solution was cooled in a refrigerator and allowed to stand for one week. This slow evaporation yielded colorless crystals of 1:1 hesperetin:iso-nicotinamide co-crystal (hereinafter “KP15”). DSC analysis showed a phase change at about 180° C.
[0189]KP15 can be characterized using XRPD, and exhibited major peaks at about the following positions: 3.6, 7.1, 10.6, 12.6, 14.2, 17.8, 19.1, 20.7, 25.0, 26.5, and 29.0 degrees. The XRPD graph for KP15 is shown in FIG. 19 of the accompanying drawings.
[0190]Hydrogen bonding occurs between two of the phenolic —OH moieties of hesperetin molecules and the following: other hesperetin molecules and the pyridyl moieties of iso-nicotinamide dimmers (OH . . . O−2.720(2) Å, OH . . . N=2.623(1) Å). Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Molar ratio | aaaaa | aaaaa |
| Stress optical coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com