Selective immunodepletion of endogenous stem cell niche for engraftment
a stem cell niche and immunodepletion technology, applied in the direction of antibody medical ingredients, drug compositions, peptides, etc., can solve the problems of poor engraftment, graft failure, microenvironment or marrow stroma dysfunction, etc., to improve the function of targeted tissue, improve the efficiency of engraftment, and eliminate endogenous stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Antibody-Based Depletion of Hematopoietic Stem Cells Empties Niches for Efficient Transplantation
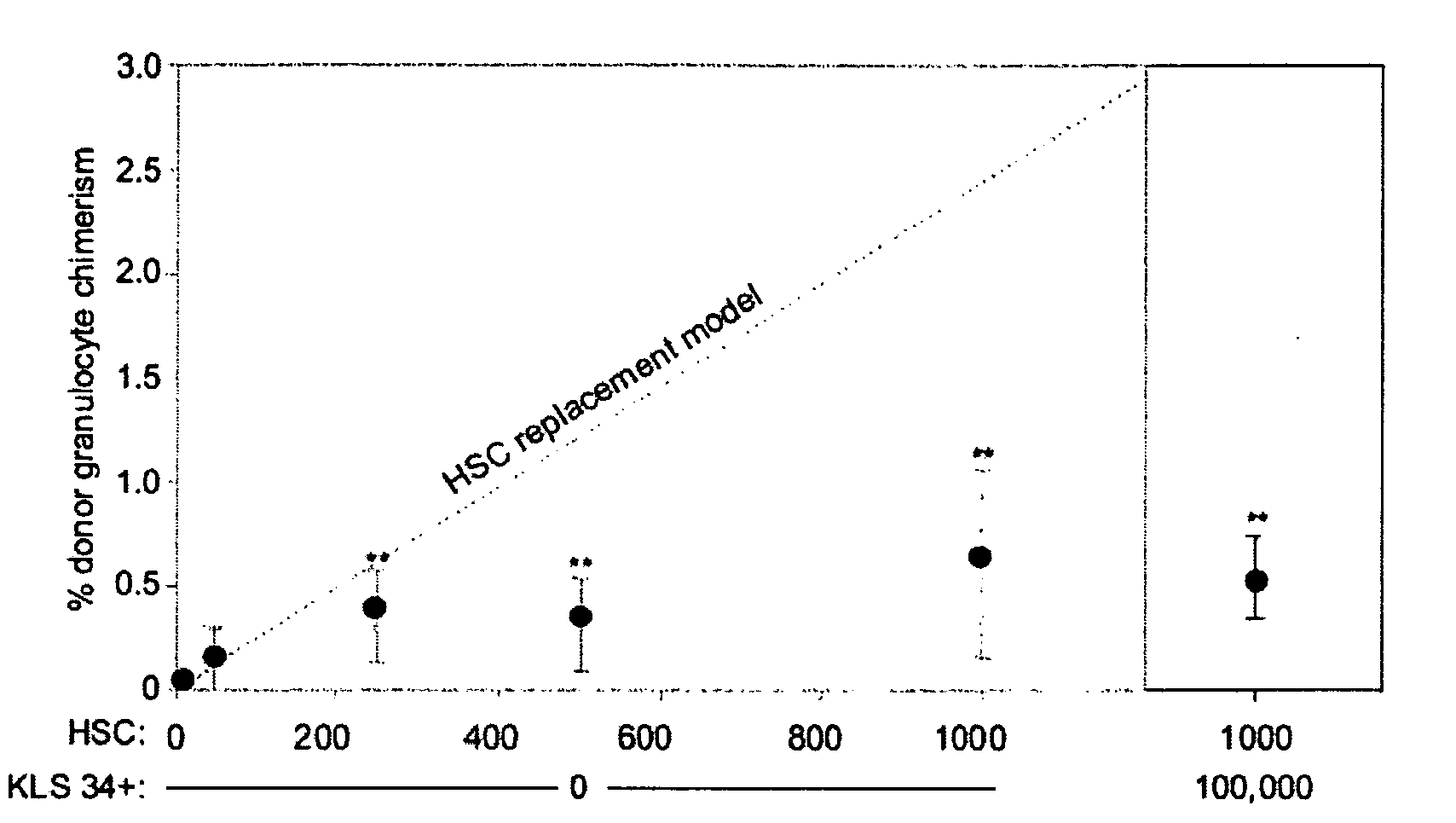
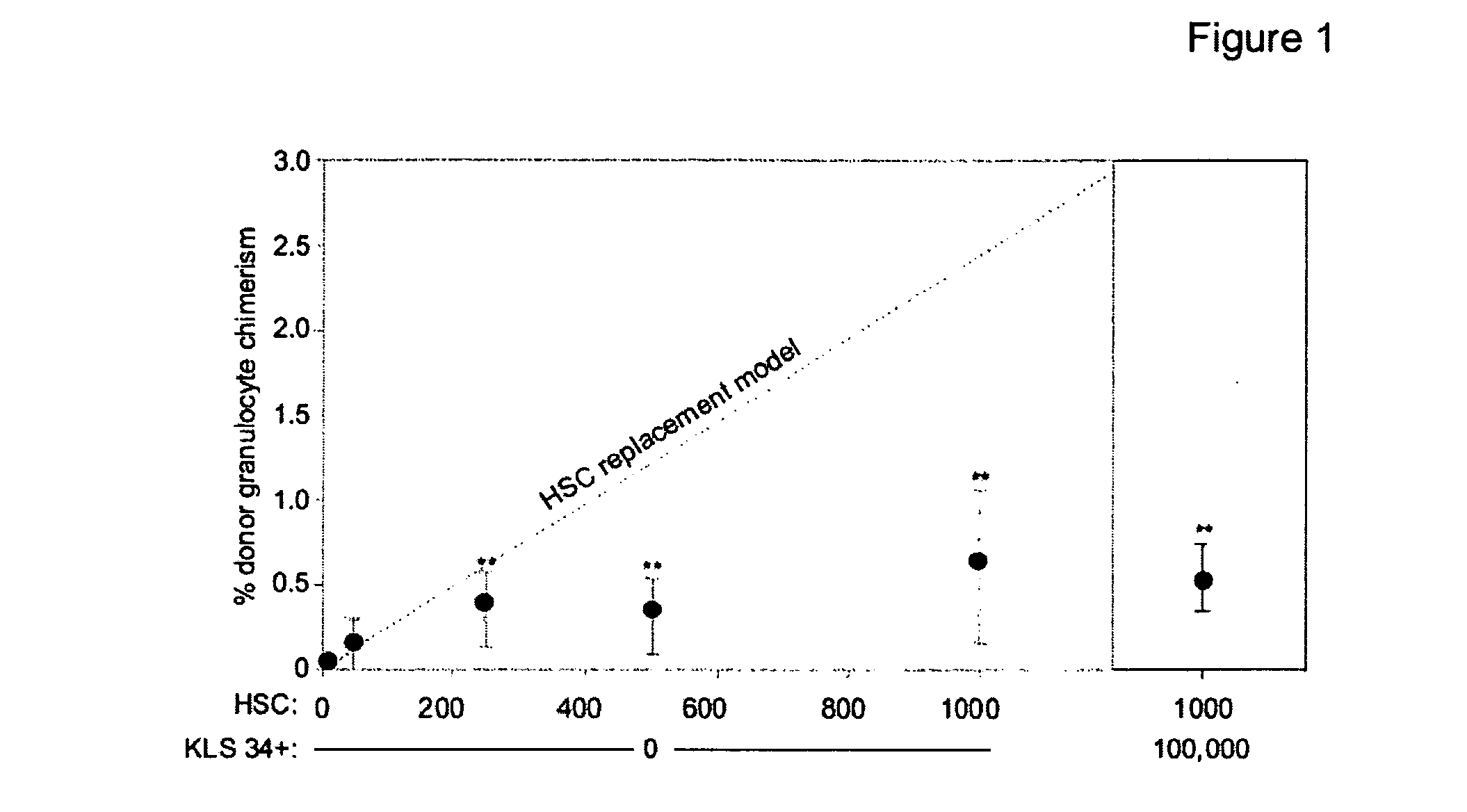
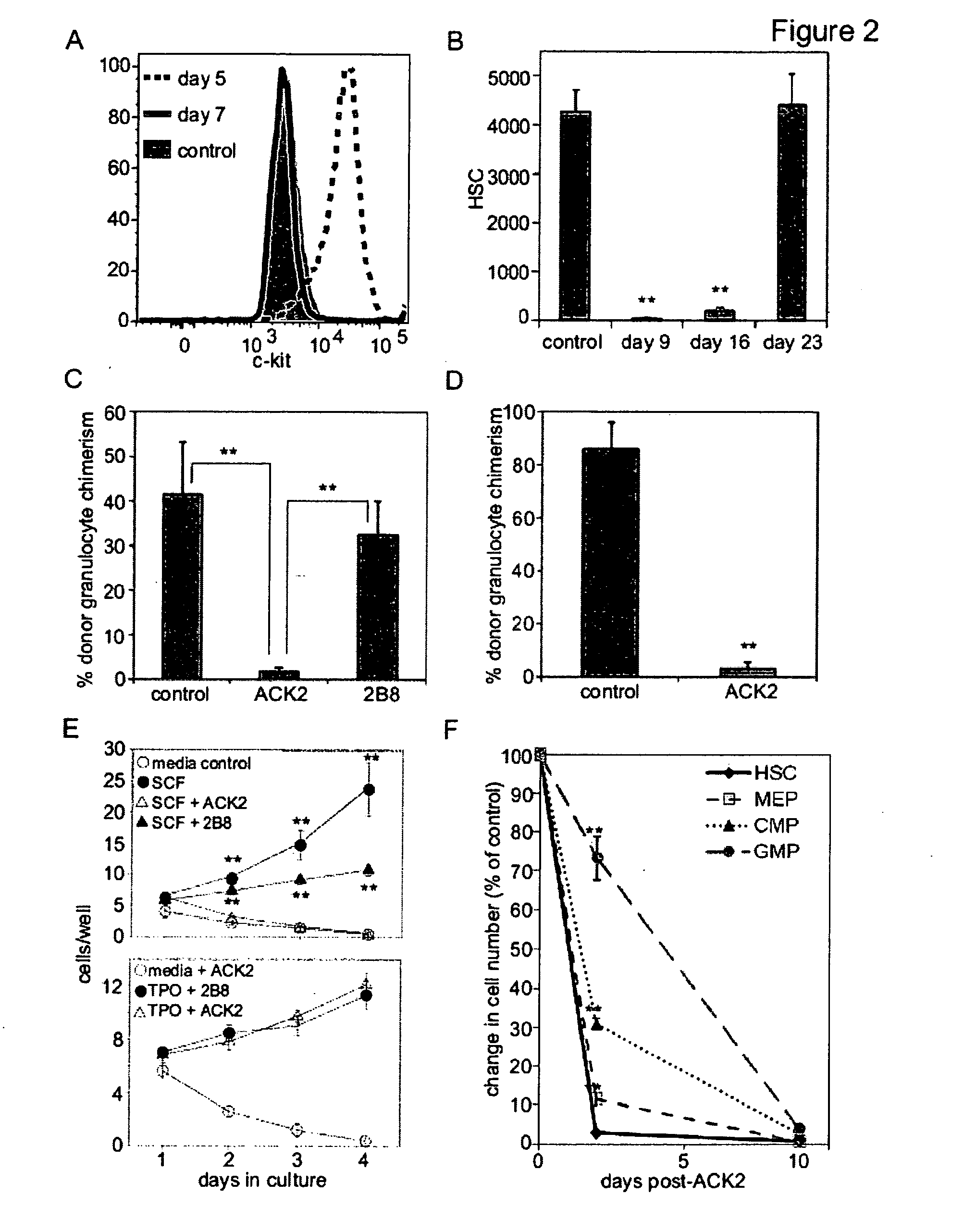
[0090]We demonstrate that administration of a depleting antibody specific for c-kit leads to the highly efficient removal of host hematopoietic stem cells (HSCs) and high levels of donor HSC chimerism following transplantation.
[0091]Upon intravenous transplantation, hematopoietic stem cells (HSCs) can home to specialized bone marrow niches, yet engraftment levels rarely exceed 0.5% following transplantation into immunodeficient recipients without toxic conditioning. Here, we provide evidence that, aside from immune barriers, donor HSC engraftment is restricted by occupancy of appropriate niches by host HSCs. Administration of ACK2, a depleting antibody specific for c-kit, led to the transient removal of >98% of endogenous HSCs and transplantation of these animals with donor HSCs led to chimerism levels of up to 90%. Extrapolation of these methods to humans may enable efficient yet mild c...
example 2
Antibody-Mediated Lymphoablation and HSC Transplantation for the Treatment of Agammaglobulinemia
[0132]We sought to determine whether transient depletion of CD4+ T cells from mice that normally reject allogeneic HSCs would allow for minor histocompatibility-mismatched HSC engraftment. Cμ− / − mice, which are a model for agammaglobulinemia and are recalcitrant to sustained donor HSC engraftment in the absence of irradiation, were treated with anti-CD4 antibody (clone GK1.5), which depletes >95% of CD4+ T cells in vivo, prior to transplantation with HSCs from GFP-transgenic mice. Short-term engraftment and B cell reconstitution was seen in all animals treated with anti-CD4 antibody, but was never seen in any of the untreated animals (FIG. 11). This chimerism, however, was lost between 8-12 weeks after transplantation as CD4+ T cell counts returned to normal. Thus, in this system, permanent transplantation tolerance was not generated by ˜0.1-0.2% HSC chimerism.
[0133]Candidate human HSC-sp...
example 3
Purified Hematopoietic Stem Cell Engraftment of Rare Niches Corrects Severe Lymphoid Deficiencies without Host Conditioning
[0135]In the absence of irradiation or other cytoreductive conditioning, endogenous hematopoietic stem cells (HSCs) are thought to fill the unique niches within the bone marrow that allow maintenance of full hematopoietic potential and thus prevent productive engraftment of transplanted donor HSCs. By transplantation of purified exogenous HSCs into unconditioned congenic histocompatible strains of mice, we show that ˜0.1-1.0% of these HSC niches are available for engraftment at any given point and find no evidence that endogenous HSCs can be displaced from the niches they occupy. We demonstrate that productive engraftment of HSCs within these empty niches is inhibited by host CD4+ T cells that recognize very subtle minor histocompatibility differences. Strikingly, transplantation of purified HSCs into a panel of severe combined immunodeficient (SCID) mice leads ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com