Sustained drug-releasing stent
a technology of stent and stent body, which is applied in the field of stent, can solve the problems of sudden death, adverse effects of stent repair or treatment, and inability to fully discharge, and achieve the effects of suppressing vascular intimal hyperplasia, and preventing the occurrence of in-stent restenosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparing example 1
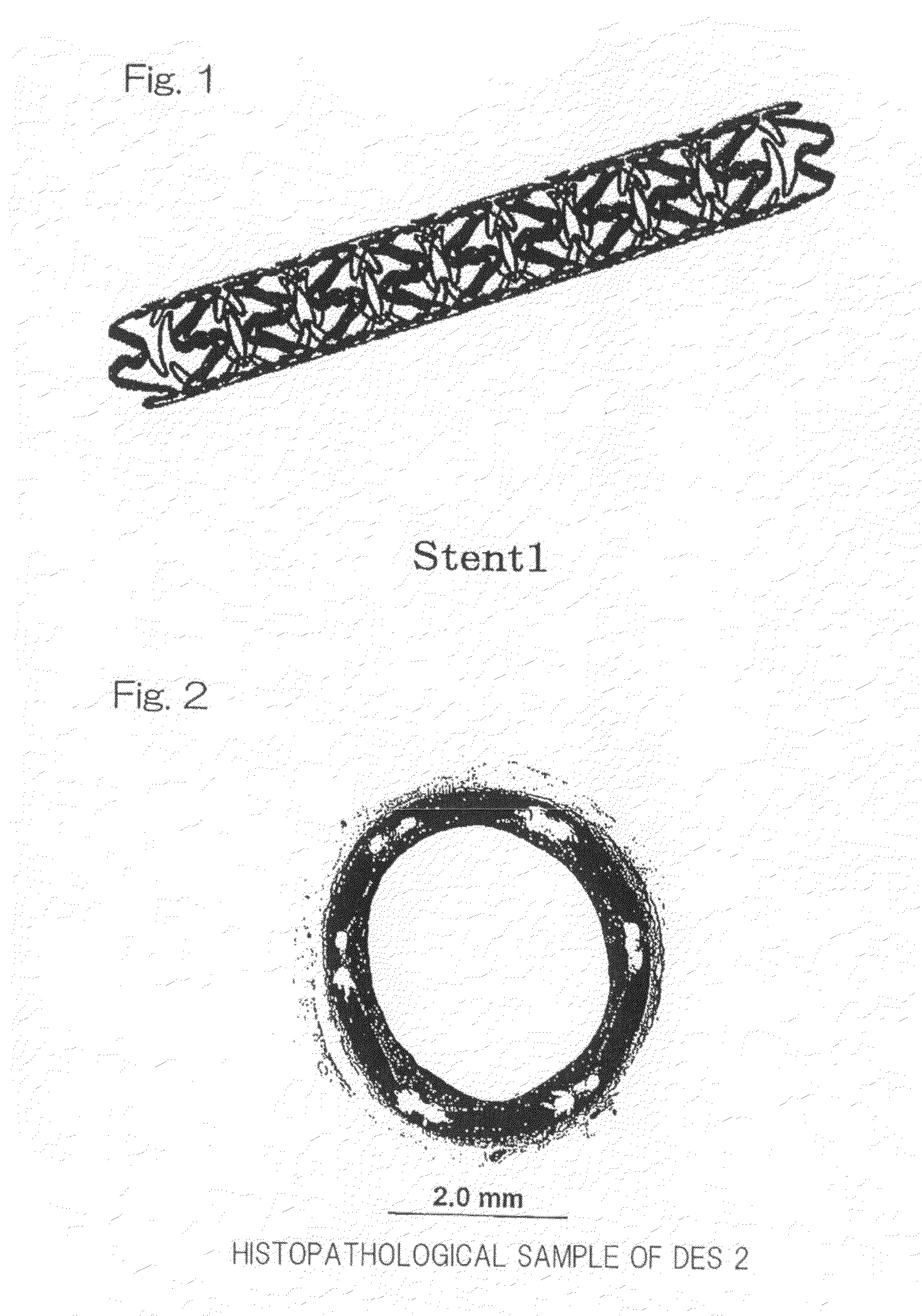
[0084]A uniformly mixed solution (coating solution) was prepared by dissolving 140 mg of argatroban and 160 mg of D, L-lactic acid / glycolic acid copolymer (mole ratio: 50 / 50) uniformly with the use of 20 mL of 2,2,2-trifluoroethanol. A stent body (hereinafter referred to as Stent 1) of such a design as shown in FIG. 1 and having the total surface are of 0.70 cm2, a length of 17 mm, an inner diameter of 3 mmφ when dilated and an outer diameter of 1.55 mmφ when processed, was mounted on a mandrel of a spray type coating apparatus. The coating solution so prepared was sprayed from a nozzle at a rate of 20 μL / min. and the stent body, held 9 mm below the nozzle and rotated at a rate of 120 rpm together with the mandrel, was reciprocatingly moved to allow the sprayed solution to be applied for about 4 minutes to a zone of the stent body ranging from one end thereof to an intermediate portion thereof. After the coating in the manner described above, the stent body was dried for 10 minutes ...
preparing example 2
[0085]A uniformly mixed solution (coating solution) was prepared by dissolving 300 mg of D, L-lactic acid / glycolic acid copolymer (mole ratio: 50 / 50) uniformly in 20 mL of chloroform. The DES1 prepared in Preparing Example 1 described above was mounted on a mandrel of a spray type coating apparatus. The coating solution so prepared was sprayed from a nozzle at a rate of 20 μL / min. and the stent body, held 9 mm below the nozzle and rotated at a rate of 120 rpm together with the mandrel, was reciprocatingly moved to allow the sprayed solution to be applied for about 100 seconds to a zone of the stent body ranging from one end thereof to an intermediate portion thereof. After the coating in the manner described above, the stent body was dried for 10 minutes with the stream of a nitrogen gas, followed by coating of the remaining zone of the stent body. The stent body, which had been completely coated over the entire surface thereof, was, after having been dried for about 1 hour, dried a...
preparing example 3
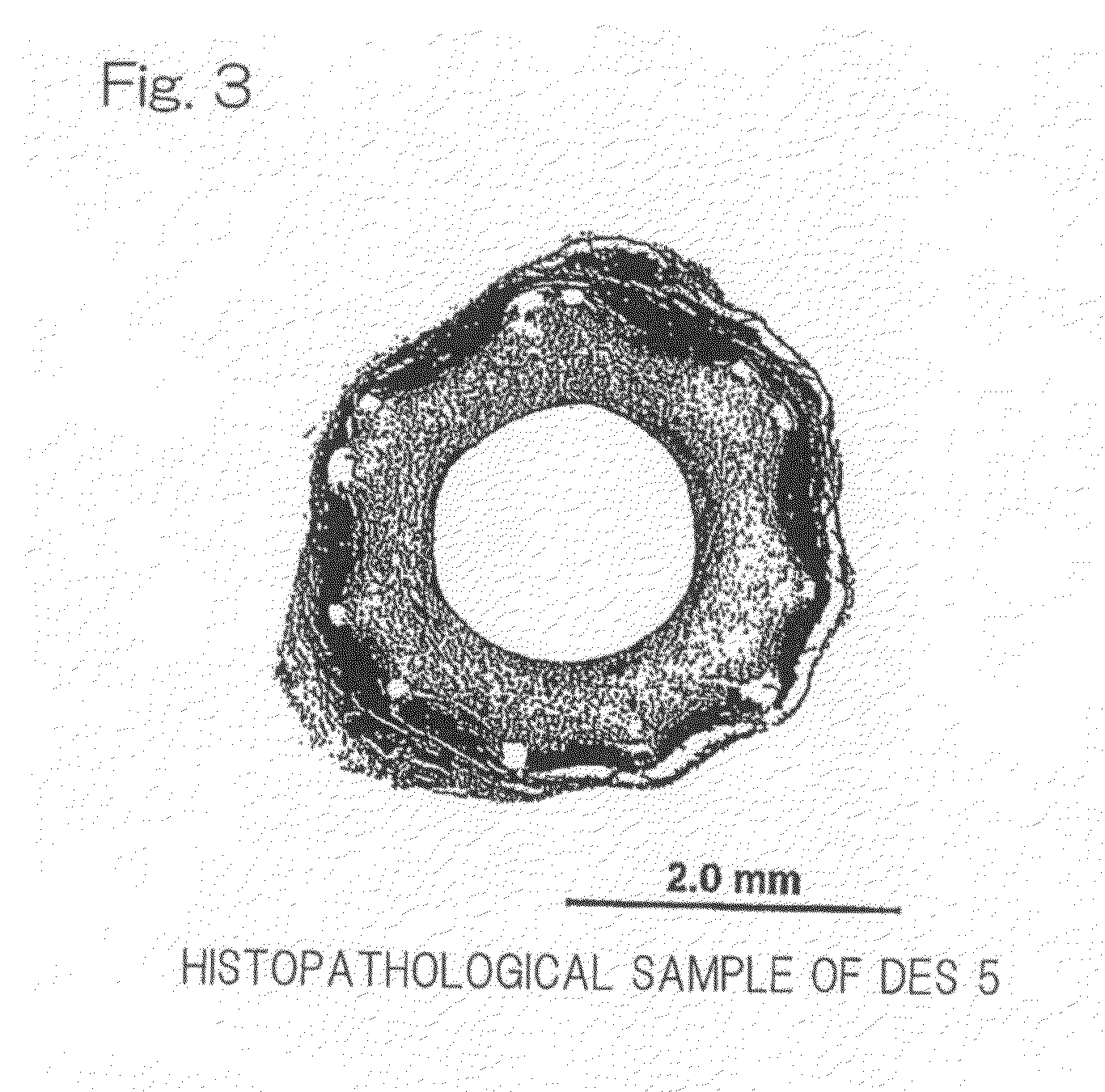
[0086]A uniformly mixed solution (coating solution) was prepared by dissolving 120 mg of argatroban and 180 mg of D, L-lactic acid / glycolic acid copolymer (mole ratio: 50 / 50) uniformly with the use of 20 mL of 2,2,2-trifluoroethanol. The Stent 1 referred to previously was mounted on a mandrel of a spray type coating apparatus. The coating solution so prepared was sprayed from a nozzle at a rate of 20 μL / min. and the Stent 1, held 9 mm below the nozzle and rotated at a rate of 120 rpm together with the mandrel, was reciprocatingly moved to allow the sprayed solution to be applied for about 4 minutes to a zone of the stent body ranging from one end thereof to an intermediate portion thereof. After the coating in the manner described above, the stent body was dried for 10 minutes with the stream of a nitrogen gas, followed by coating of the remaining zone of the stent body. The stent body, which had been completely coated over the entire surface thereof, was, after having been dried fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com