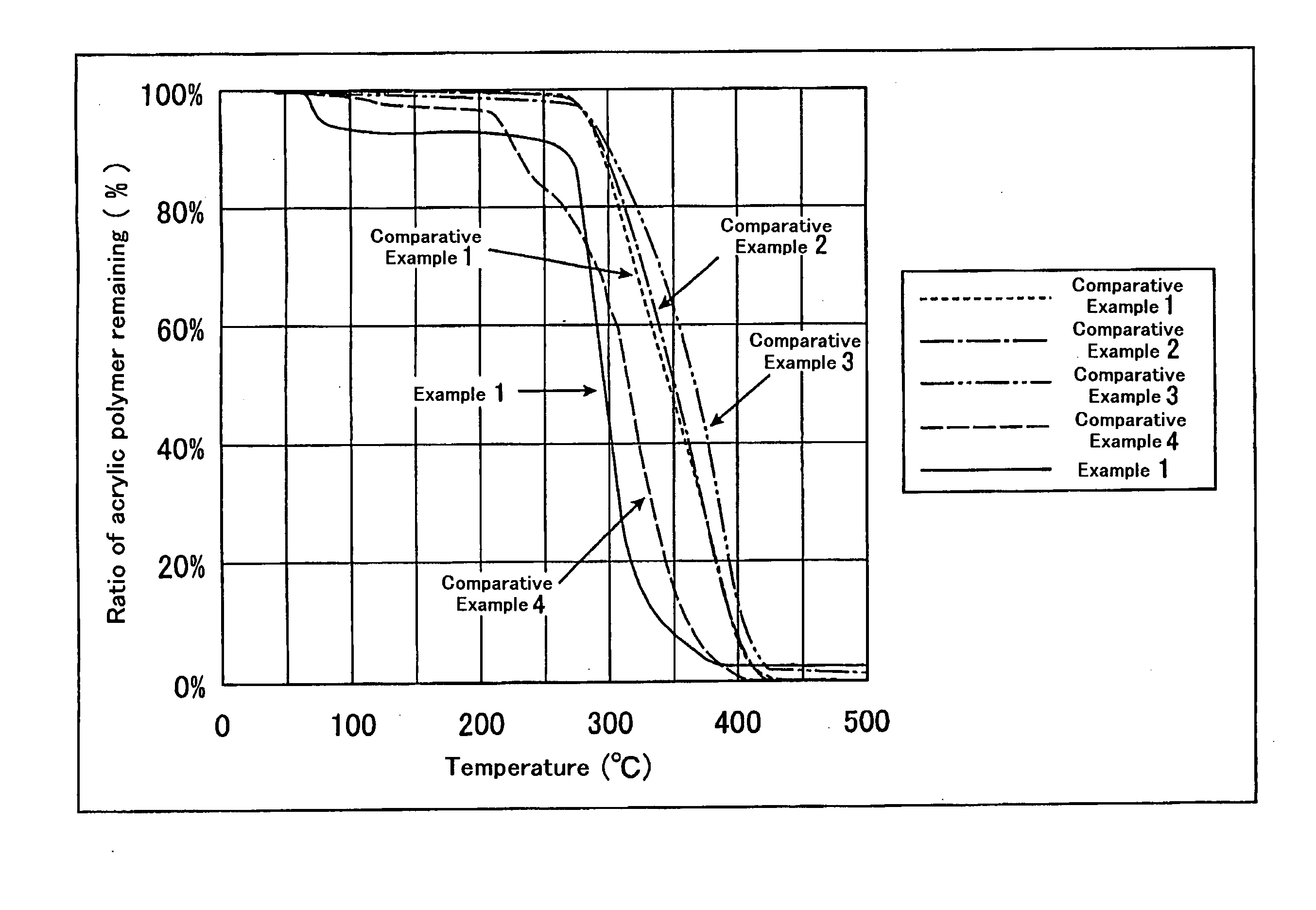
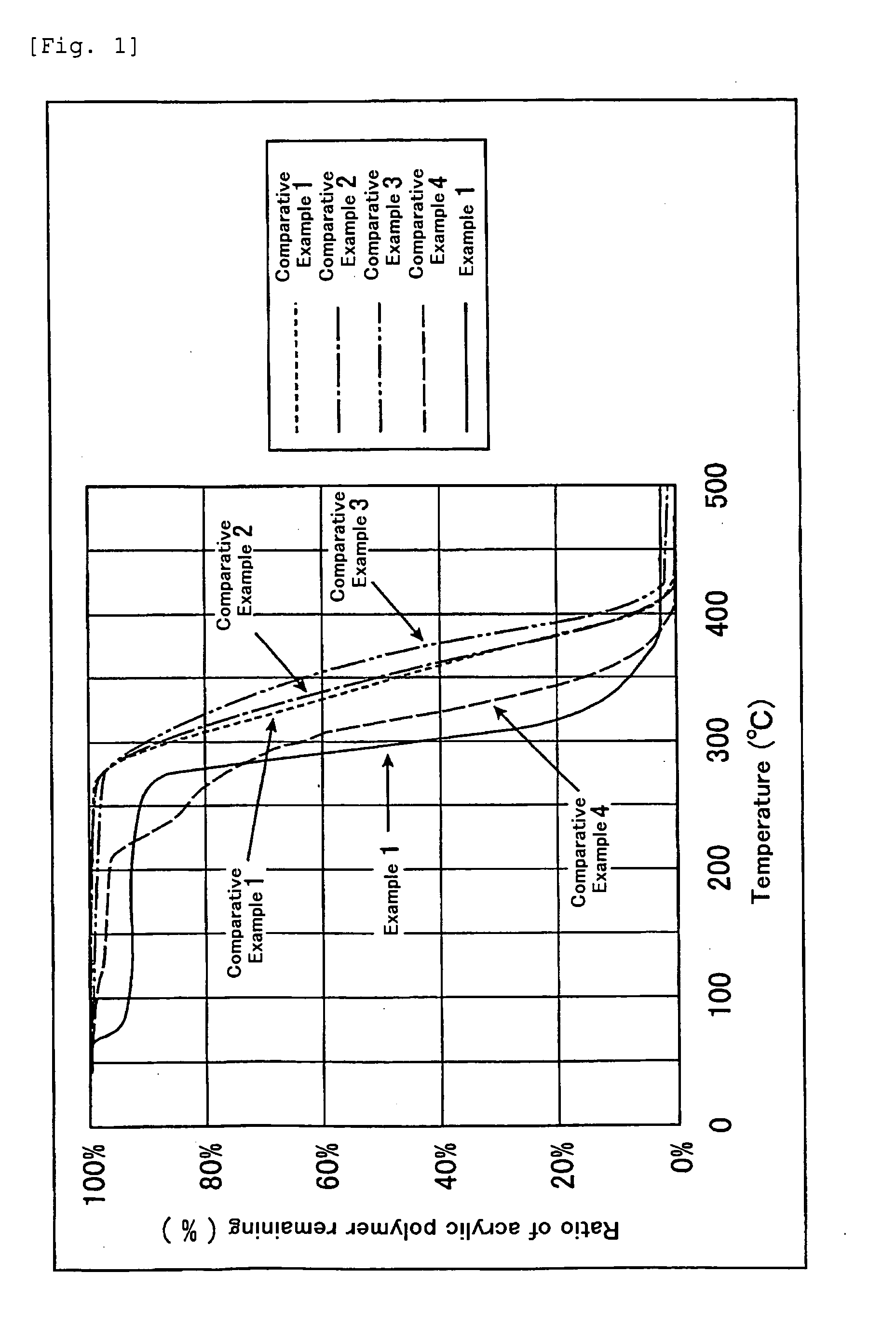
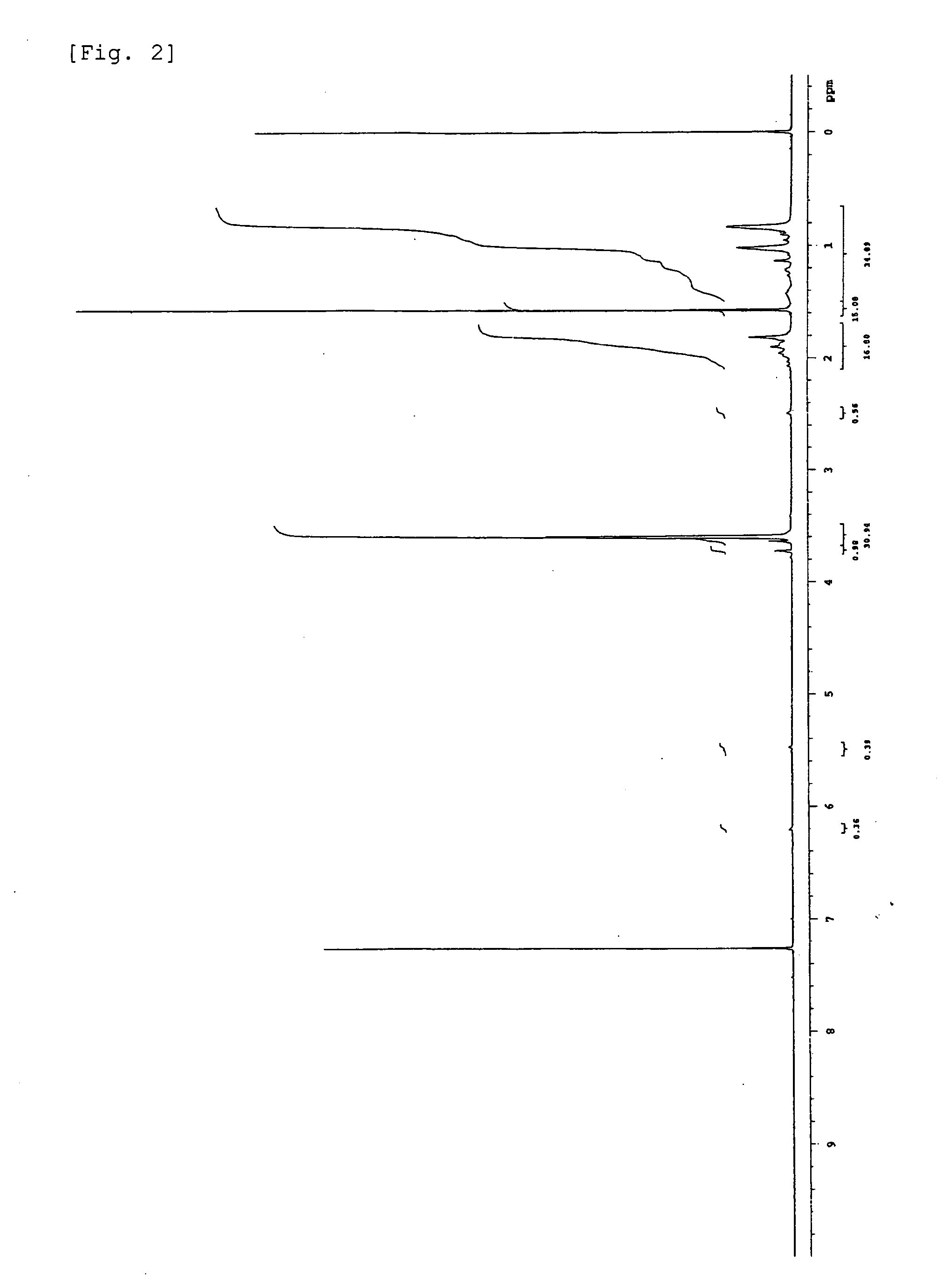
Terminally modified acrylic polymer and method for producing terminallly modified acrylic polymer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(Production of Acrylic Polymer)
[0052]30 mol of methyl methacrylate (manufactured by Wako Pure Chemical Industries, Ltd.) as a monomer was added to 1 mol of an organic bismuth compound CH3C(CH3)(Bi(CH3)2)COOCH3 as a living radical polymerization initiator, and then, the resulting mixture was heated to 100° C. while stirring the mixture with a stirrer and maintained at this temperature for 3 hours. After the completion of a reaction, the reaction solution was dissolved in 15 mL of α,α,α-trifluorotoluene. To the resulting solution, 0.2 g of 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO manufactured by Aldrich Chemical Co.) was further added and the resulting mixture was reacted at 80° C. for 1 hour. After the completion of a reaction, the resulting solution was charged into 250 mL of hexane (manufactured by Wako Pure Chemical Industries, Ltd.) being stirred. Thereafter, a precipitated polymer was filtered under suction and dried to obtain a terminally modified acrylic polymer (conversion...
example 2
(Production of Acrylic Polymer)
[0056]30 mol of isobutyl methacrylate (manufactured by Wako Pure Chemical Industries, Ltd.) as a monomer was added to 1 mol of an organic bismuth compound CH3C(CH3)(Bi(CH3)2)COOCH3 as a living radical polymerization initiator, and then, the resulting mixture was heated to 100° C. while stirring the mixture with a stirrer and maintained at this temperature for 3 hours. After the completion of a reaction, the reaction solution was dissolved in 15 mL of α,α,α-trifluorotoluene. To the resulting solution, 0.2 g of 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO manufactured by Aldrich Chemical Co.) was further added and the resulting mixture was reacted at 80° C. for 1 hour. After the completion of a reaction, the resulting solution was charged into 250 mL of hexane (manufactured by Wako Pure Chemical Industries, Ltd.) being stirred. Thereafter, a precipitated polymer was filtered under suction and dried to obtain a terminally modified acrylic polymer (conversi...
example 3
(Production of Acrylic Polymer)
[0059]A mixture solution of 15 mol of isobutyl methacrylate (manufactured by Wako Pure Chemical Industries, Ltd.) and 15 mol of isobutyl methacrylate (manufactured by Wako Pure Chemical Industries, Ltd.) as a monomer was added to 1 mol of an organic bismuth compound CH3C(CH3)(Bi(CH3)2)COOCH3 as a living radical polymerization initiator, and then, the resulting mixture was heated to 100° C. while stirring the mixture with a stirrer and maintained at this temperature for 3 hours. After the completion of a reaction, the reaction solution was dissolved in 15 mL of α,α,α-trifluorotoluene. To the resulting solution, 0.2 g of 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO manufactured by Aldrich Chemical Co.) was further added and the resulting mixture was reacted at 80° C. for 1 hour. After the completion of a reaction, the resulting solution was charged into 250 mL of hexane (manufactured by Wako Pure Chemical Industries, Ltd.) being stirred. Thereafter, a pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pressure | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com