Combination Immunogene Therapy
a combination immunogene and tumor technology, applied in the field of tumor immunotherapy, can solve the problems of affecting affecting the survival rate of cancer patients, and affecting the success rate of chemotherapeutics as anticancer agents, so as to increase the immunogenicity of human tumors and effective cancer immunotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0104]Construction of Mono- and Bi-Cistronic Retroviral Vectors and Packaging of Recombinant Virus
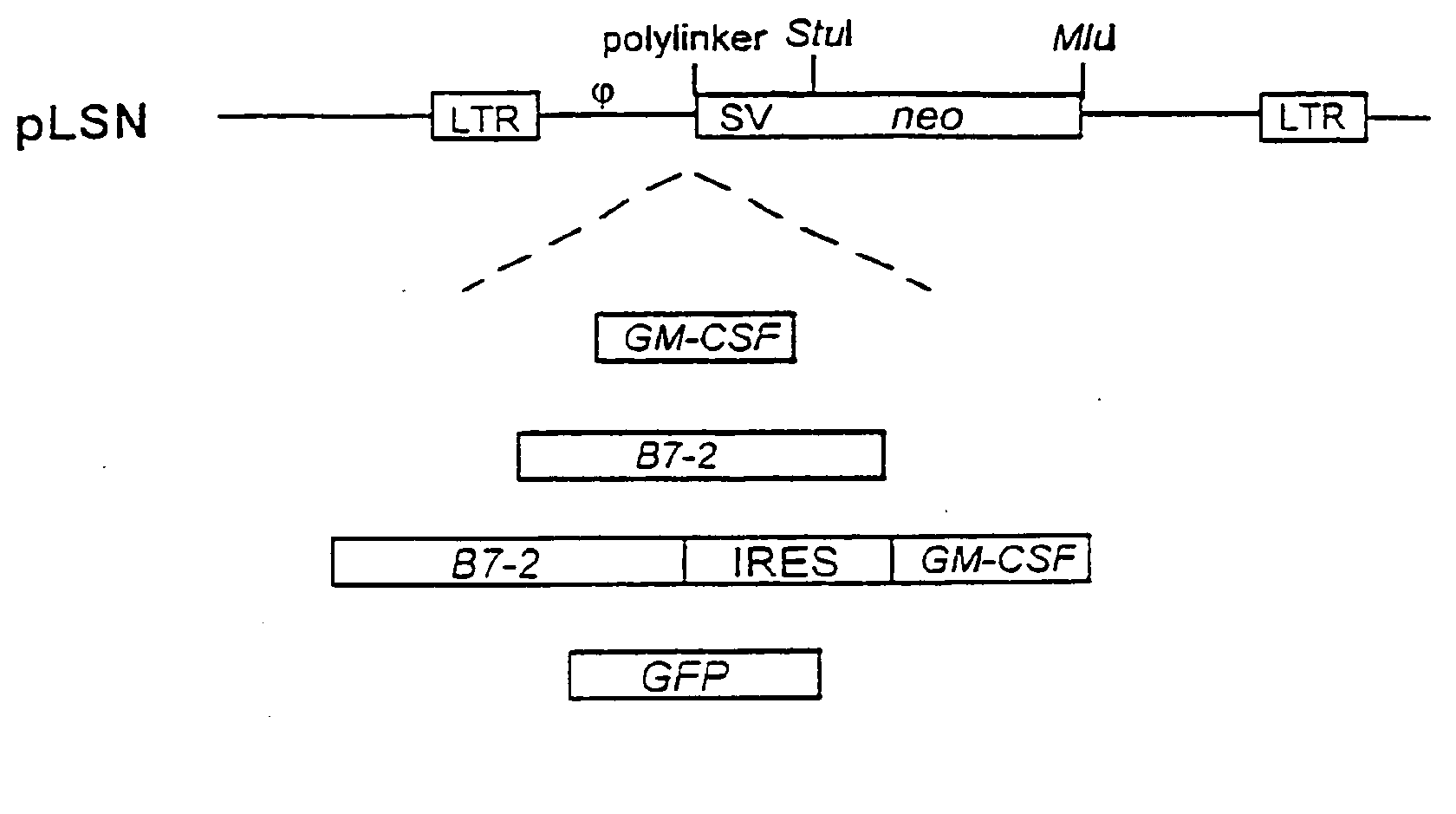
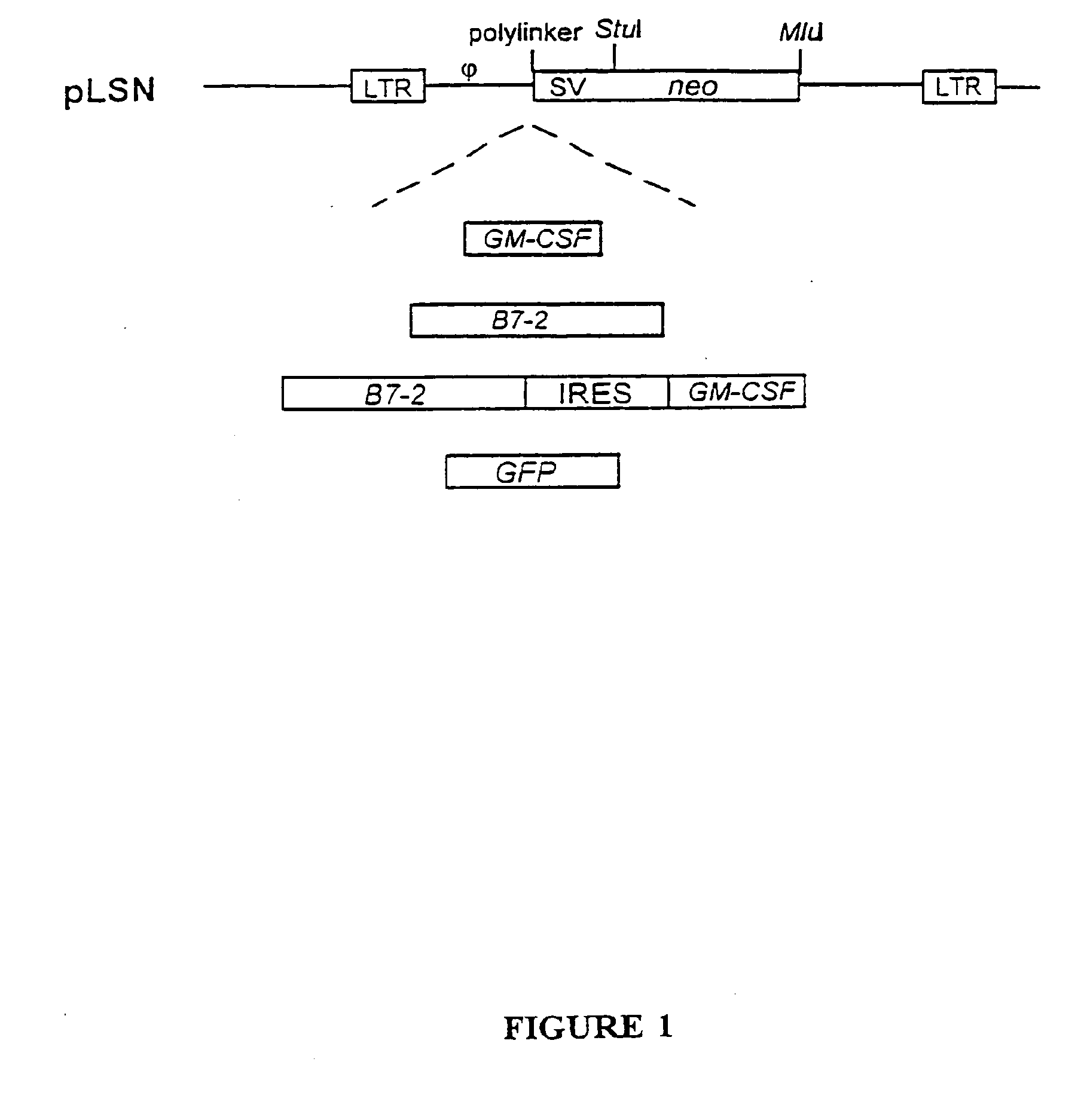
[0105]Retroviral gene therapy vectors were constructed that contained the genes for GM-CSF, B7-2, GM-CSF and B7-2, or GFP gene as shown schematically in FIG. 1. FIG. 1 provides a schematic showing the map of the parental vector (pLSN), pLSNB70 (encodes human B7-2), pLSNGM1 (encodes human GM-CSF), pLSN-BG9 (encodes both B7-2 and GM-CSF) and pLSN-GFP (encodes GFP).
[0106]All genes were cloned into the polylinker region of the MLV-based pLSN plasmid [Robinson et al. (1995) Gene Therapy 2:269 and co-pending application Ser. No. 08 / 336,132]. Therapeutic genes were inserted into pLSN such that their expression was under the control of the retroviral LTR (long terminal repeat) whereas a neomycin-resistance gene was under the control of an internal SV40 promotor. The vectors lacked the gag, pol, or env genes necessary for retroviral packaging in order to render them replication incompetent. Thes...
example 2
[0128]Expression of B7-2 and GM-CSF in the Human Glioblastoma Cell Line D54MG
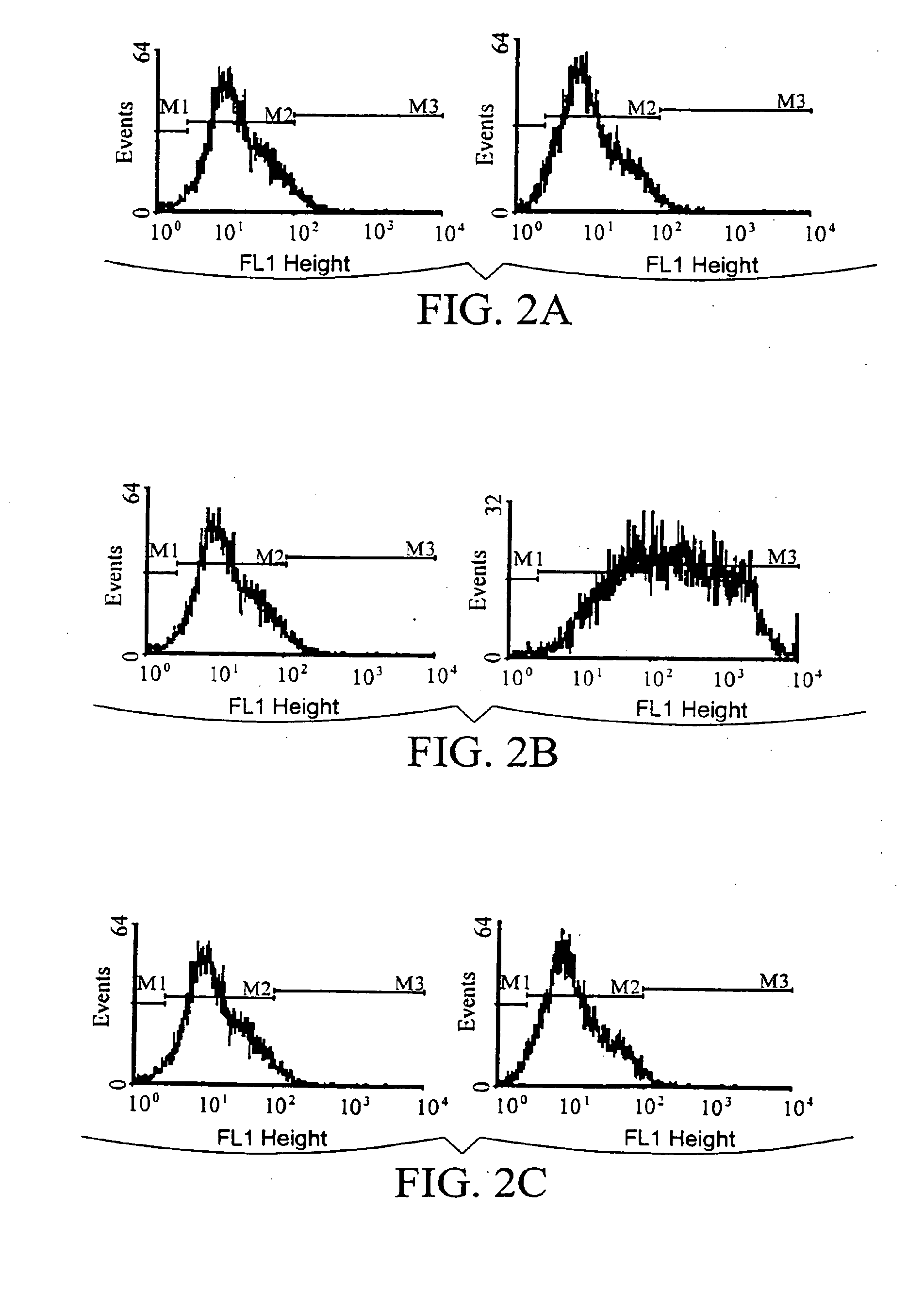
[0129]Viral particles containing recombinant retroviral genomes encoding either GM-CSF, B7-2, B7-2 / GM-CSF or GFP were used to transduce the human glioblastoma cell line D54MG.
[0130]a) Growth Of D54MG In Tissue Culture
[0131]The human glioblastoma cell line D54MG [obtained from Dr. D. Bigner, Duke University, Durham, N.C.; Bigner et al. (1981) J. Neuropathol. Exp. Neurol. 40:201] was cultured in Dulbucco's Modified Eagle's Media (DMEM) with 10% fetal bovine serum (Gibco), 0.2 units / ml penicillin-streptomycin solution (Sigma), and 0.2 mM glutamate at 37° C. in a humidified atmosphere containing 5% CO2.
[0132]b) Retroviral Transduction
[0133]Frozen virus stock was thawed at 37° C. Polybrene was added to the thawed virus solution at a final concentration of 4 μg / ml. Culture media was added to the virus solution to bring the final volume to 1.5 ml. D54MG cells in logarithmic growth phase in T25 flasks (approximatel...
example 3
[0145]Tumor Growth Efficiency and Human PBL Reconstitution in SCID / Beige and SCID / nod Mice
[0146]This example describes the reconstitution of SCID / nod and SCID / beige mice with human PBL and the engraftment of human tumor cells in these mouse strains.
[0147]a) Animals and Human PBL Reconstitution
[0148]For most experiments, four to five week old female C.B-17-SCID-beige mice were purchased from Taconic (Germantown, N.Y.). For the first vaccination / challenge experiment, four to five week old female SCID / nod mice were obtained from Dr. L. Pilarski (Cross Cancer Institute, University of Alberta, Edmonton, Alberta). The mice were maintained in filtered cages in a virus free environment and received cotrimoxazole in their drinking water twice per week.
[0149]Hu-PBL-SCID mouse reconstitution was carried out as previously described [Zhang et al. (1996) Proc. Natl. Acad. Sci. USA 93:14720]. Briefly, each mouse was intraperitoneally (IP) injected with 2-3×107 PBLs resuspended in 0.5 ml of Hanks' ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com