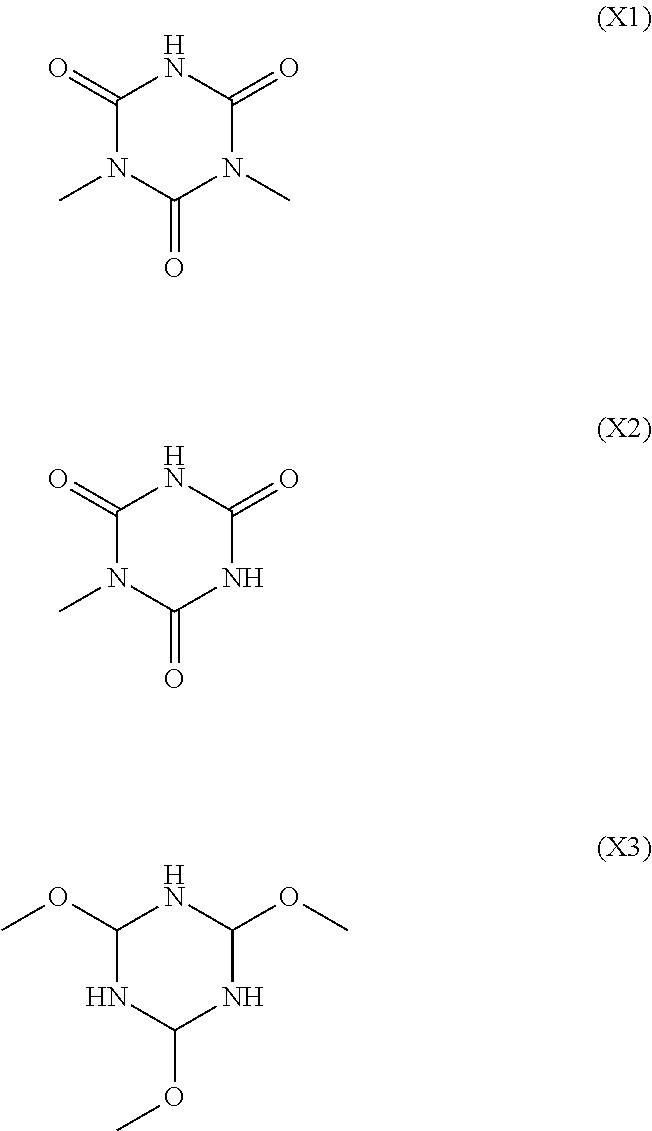
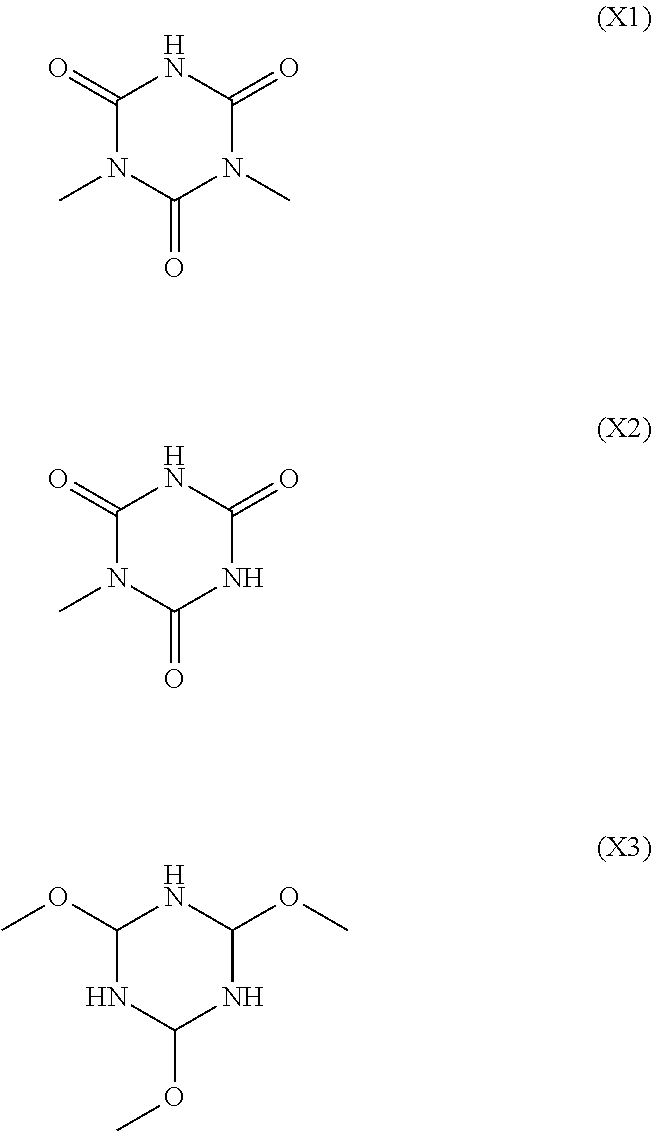
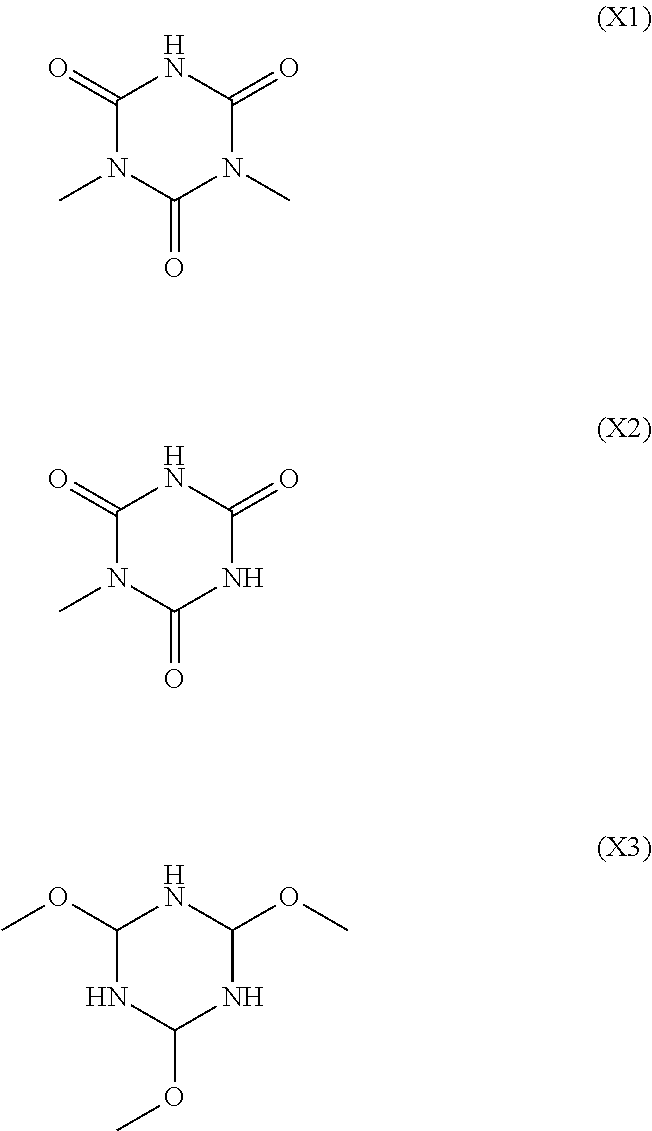
Alkali-developable curable composition, insulating thin film using the same, and thin film transistor
a technology of alkali-developable curable composition and thin film, which is applied in the field of polysiloxane compounds, can solve the problems of lack of reliability, inability to meet requirements, and decreases in heat resistance and light resistance, and achieves superior electrical insulation properties and heat-resistant transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0290]100 g of toluene and 57.49 g of 1,3,5,7-tetramethylcyclotetrasiloxane were placed in a 500 mL four-mouth flask followed by replacing the gas phase with nitrogen and heating and stirring at an internal temperature of 105° C. A mixed liquid of 10.0 g of diallyl isocyanuric acid, 70.0 g of 1,4-dioxane and 0.0186 g of a xylene solution of platinum-vinylsiloxane complex (containing 3% by weight of platinum) was added dropwise over the course of 30 minutes. Six hours after completion of dropping, the degree of reaction of allyl groups was confirmed to be 95% or more by 1H-NMR and the reaction was terminated by cooling. Unreacted 1,3,5,7-tetramethylcyclotetrasiloxane and toluene were distilled off under reduced pressure to obtain a colorless, clear liquid referred to as “Reaction product A”.
[0291]20 g of toluene and 10 g of “Reaction product A” were placed in a 100 mL four-mouth flask followed by replacing the gas phase with nitrogen, heating at an internal temperature of 105° C., ad...
example 2
[0292]20 g of toluene and 10 g of “Reaction product A” were placed in a 100 mL four-mouth flask followed by replacing the gas phase with nitrogen, heating at an internal temperature of 105° C., adding a mixture of 3.7 g of vinyltrimethoxysilane and 3.0 g of toluene thereto, and confirming the degree of reaction of vinyl groups to be 95% or more by 1H-NMR 3 hours after addition. The reaction liquid was then cooled to obtain “Reaction product 2”.
example 3
[0293]20 g of toluene and 10 g of “Reaction product A” were placed in a 100 mL four-mouth flask followed by replacing the gas phase with nitrogen, heating at an internal temperature of 85° C., adding a mixture of 3.2 g of allyl methacrylate, 3.0 g of toluene and 0.01 g of a xylene solution of platinum-vinylsiloxane complex (containing 3% by weight of platinum) thereto, and confirming the degree of reaction of allyl groups to be 95% or more by 1H-NMR 7 hours after addition. The reaction liquid was then cooled to obtain “Reaction product 3”.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com