Emission ratiometric indicators of phosphoinositides
a technology of phosphoinositide and emission ratio, applied in the field of monitoring of phosphoinositide, can solve the problems of limiting the ability of scientists to examine different pools of pis, no reliable methods available, and inability to target subcellular locations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0069]Methods
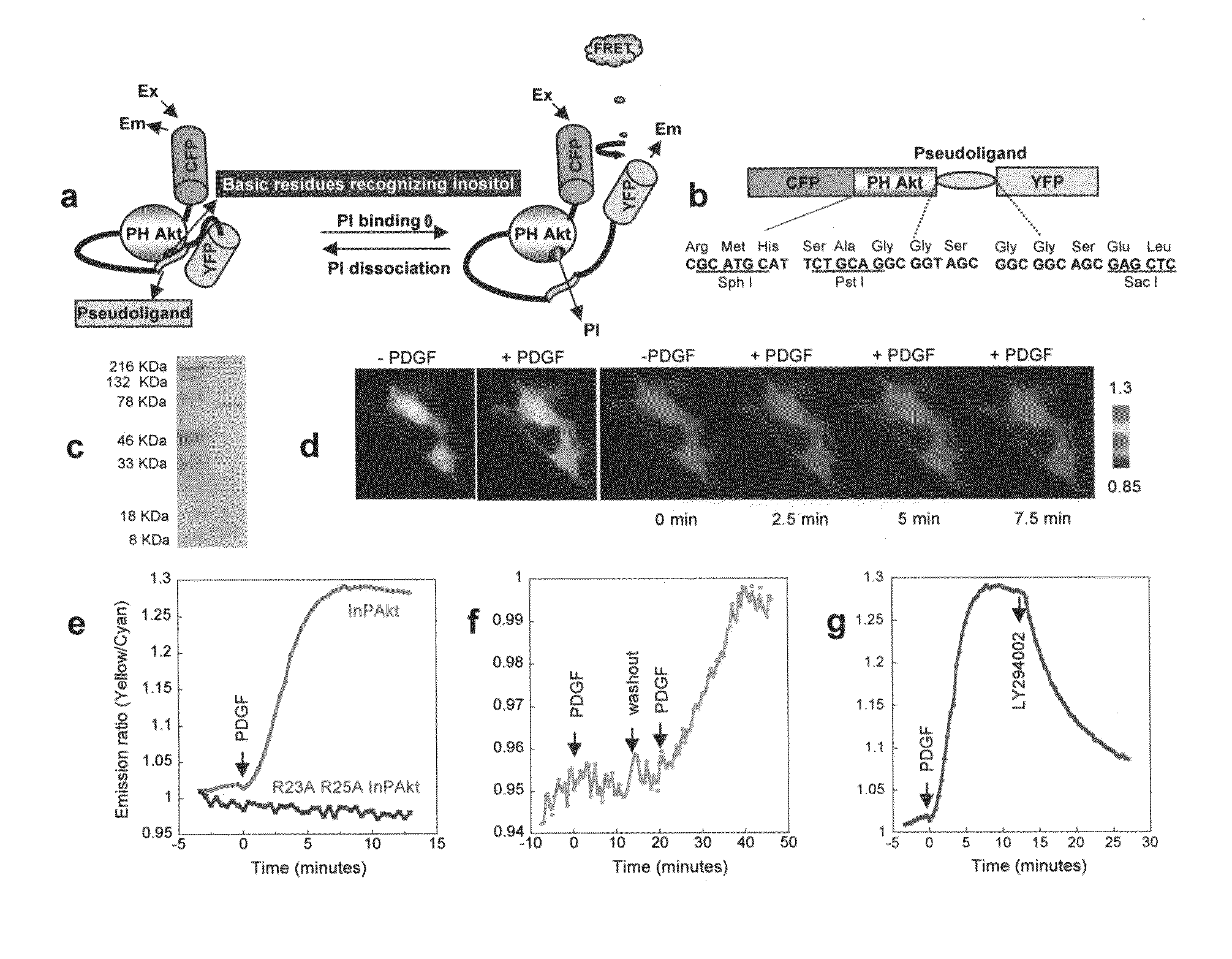
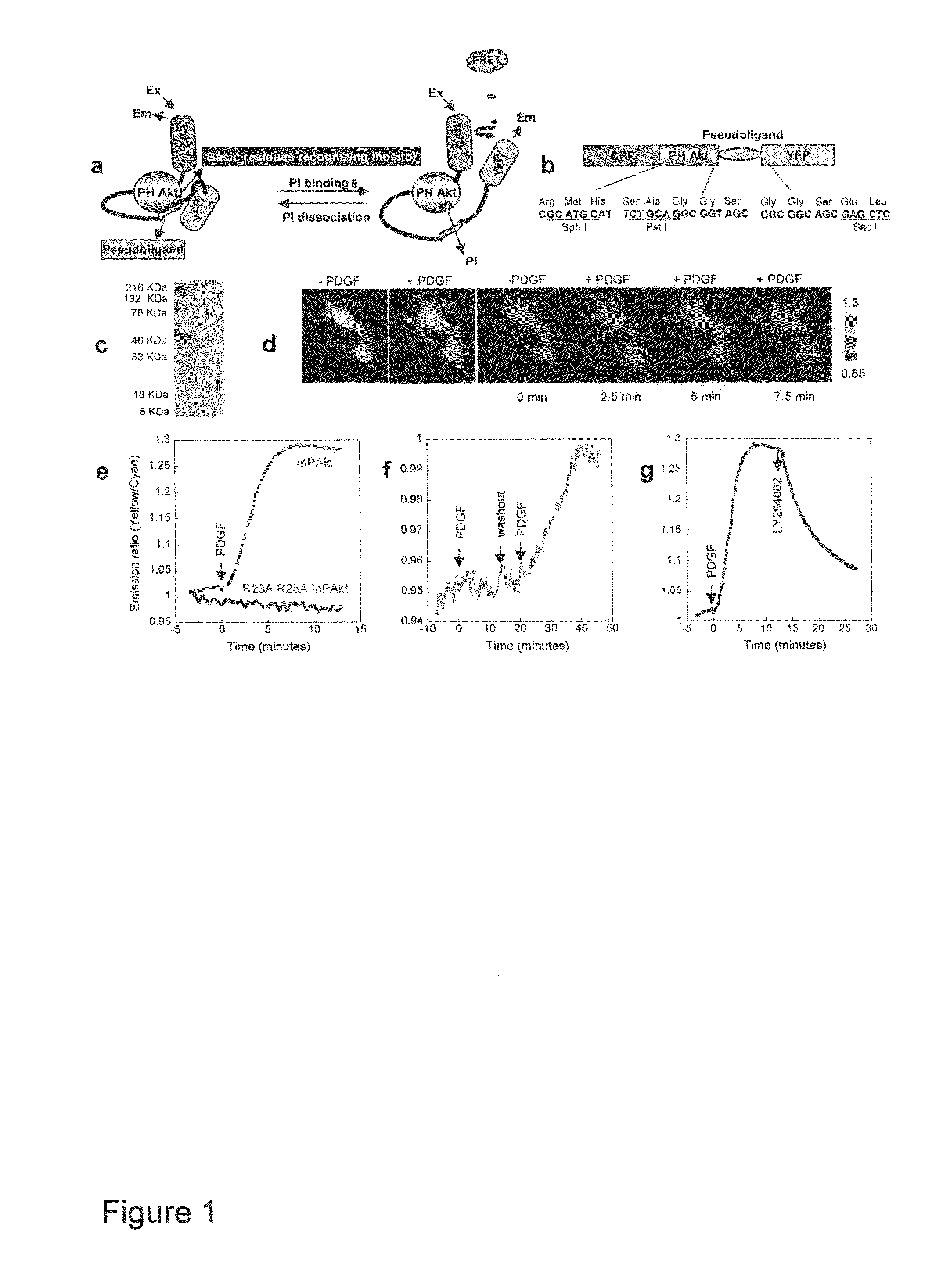
[0070]Reporter construction. The Akt / PKB PH domain (1-164) was created by PCR using full length human Akt (SEQ ID NO:1) as the template. The pseudoligand peptide sequence VAEEEDDEEEDEDD (SEQ ID NO:3) was inserted between the C-terminus of PH domain and N-terminus of improved versions of YFP, Citrine (16) or Venus (17). Double mutation R23A / R25A was incorporated by the QUICKCHANGE™ method (Stratagene) (FIG. 1). The constructs were first generated in pRSET B (Invitrogen) and subcloned into modified pcDNA3 (Invitrogen) behind a Kozak sequence for mammalian expression. For plasma membrane targeting of InPAkt, the sequence KKKKKSKTKCVIM (SEQ ID NO:4) was used. For nuclear targeting, the nuclear localization signal (NLS) PKKKRKVEDA (SEQ ID NO:5) was attached to the C terminus of the Venus-containing construct.
[0071]Cell Culture. HEK293 and NIH3T3 cells were plated onto sterilized glass coverslips in 35-mm dishes and grown to 50-90% confluency in DMEM (10% FBS) at 37° C. with ...
example 2
[0073]Development of a FRET Based Phosphoinositide Indicator
[0074]Design of the reporter. To measure the spatiotemporal dynamics of PIs in living cells, we employed a general molecular design in which binding of PIs in the “sensing unit” of the indicator can be translated into a change in the “reporting unit.” A pair of fluorescent proteins that can undergo fluorescence resonance energy transfer (FRET), enhanced cyan fluorescent protein (ECFP) and improved versions of yellow fluorescent proteins (YFP) namely Citrine (16) or Venus (17), was employed as the reporting unit (FIGS. 1A, 1B). These two fluorophores can be genetically fused to a conformationally responsive element, the conformational change of which alters the relative distance and / or orientation of the two fluorophores and generates a change in the emission ratio. Recently many such reporters have been developed for measuring signaling molecules like Ca2+, cGMP, cAMP, or signaling events such as protein phosphorylation (14...
example 3
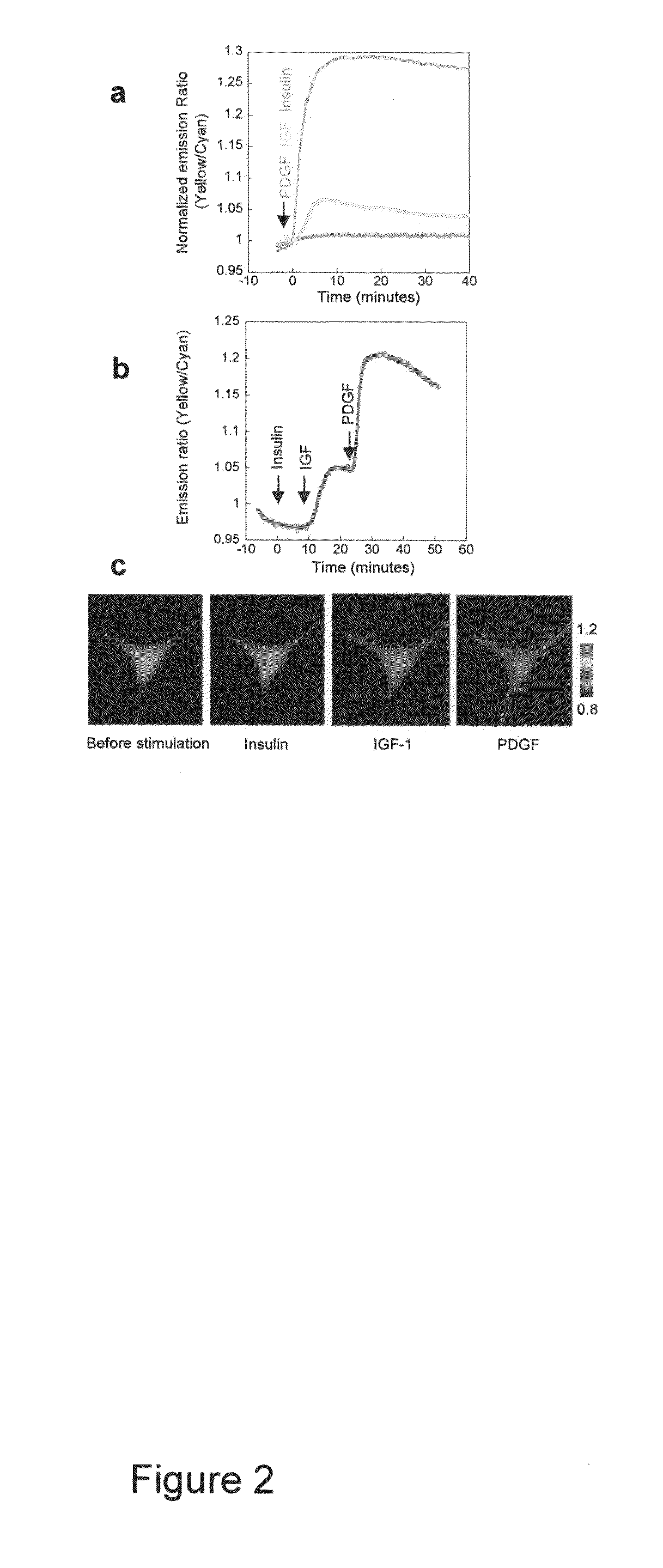
[0081]Differential Dynamics Stimulated by Different Growth Factors
[0082]Although PI3K is a crucial regulatory component shared by various growth factor pathways, their differential coupling to PI3K isoforms (23), and distinct modes of negative regulation (24), could lead to significant difference in PI dynamics, thereby differentiating downstream signaling. To compare the accumulation of PIP3 and PI(3,4)P2 induced by the activation of different tyrosine kinase receptor pathways, we applied three different ligands, namely insulin, insulin-like growth factor 1 (IGF-1) and PDGF, to serum-starved NIH3T3 cells expressing InPAkt. As shown above, PDGF stimulation produced an acute response that reached a plateau of 25.4±3.7% ratio increase in 5-9 min (t1 / 2=3.45±0.65 min) (FIG. 2A). In contrast, addition of 50 nM IGF-1 to activate insulin like growth factor receptor (IGF-IR) produced a more gradual response of 6.5±0.8% (n=5) in 10-12 min (1112=5.4±0.4 min) (FIG. 2A). Finally, stimulation wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| green fluorescent protein | aaaaa | aaaaa |
| red fluorescent protein | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com