PON polypeptides, polynucleotides encoding same and compositions and methods utilizing same
a technology of pon1 and polynucleotides, applied in the field of pon polypeptides and polynucleotides encoding same, can solve the problems of low yield of sera-purified pon1, deficiency of pon1 enzyme, and high risk of damage, and achieve the effect of increasing substrate specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning and Expression of Wild-Type PON1 Genes in E. coli
[0265]Materials and Experimental Procedures
[0266]Cloning of PON1 genes—The plasmid pGex-6p-2 containing the hPON1 gene ((Reddy et al., 2001) kindly provided by Srinivasa T. Reddy, UCLA) was used as a template for PCR amplification with a back primer (pET20-hPON1-bc; Table 1, below) that introduces an NcoI restriction site at the ATG initiation codon, and a forward primer (pET20-hPON1-fo, Table 1, below) annealing downstream to the NotI site. The resulting fragment was digested and cloned into pET20b and pET32b (Novagen,) using the NcoI and NotI sites. For cloning into pET43b, pET43-hPON1-bc (Table 1, below) was used as a back primer annealing downstream to the ATG initiation codon and appending an SpeI restriction. For cloning to pMAL (NEB), a back primer appending an EcoRI site (pMAL-hPON1-bc, Table 1, below), and a forward primer (pMAL-hPON1-fo, Table 1, below) annealing to the hPON1 gene upstream to the stop codon and appe...
example 2
Directed Evolution of Soluble PON1 Variants
[0272]Once conditions, under which high amounts of PON1 were expressed to form inclusion bodies in equilibrium with low amount of soluble and active PON1, were at hand (see Example 1, above), these served as a starting point for the directed evolution of highly soluble recombinant PON1 mutants.
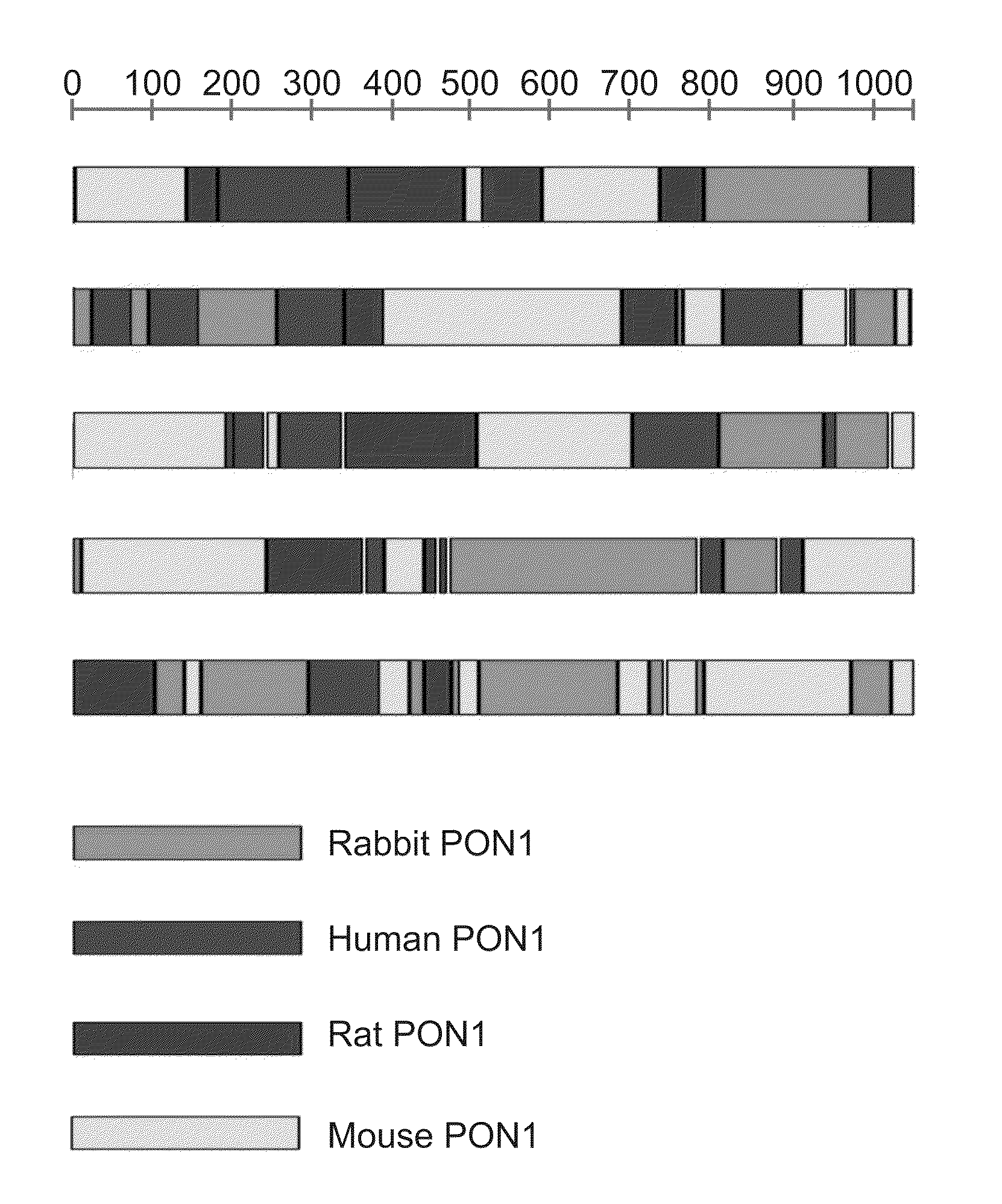
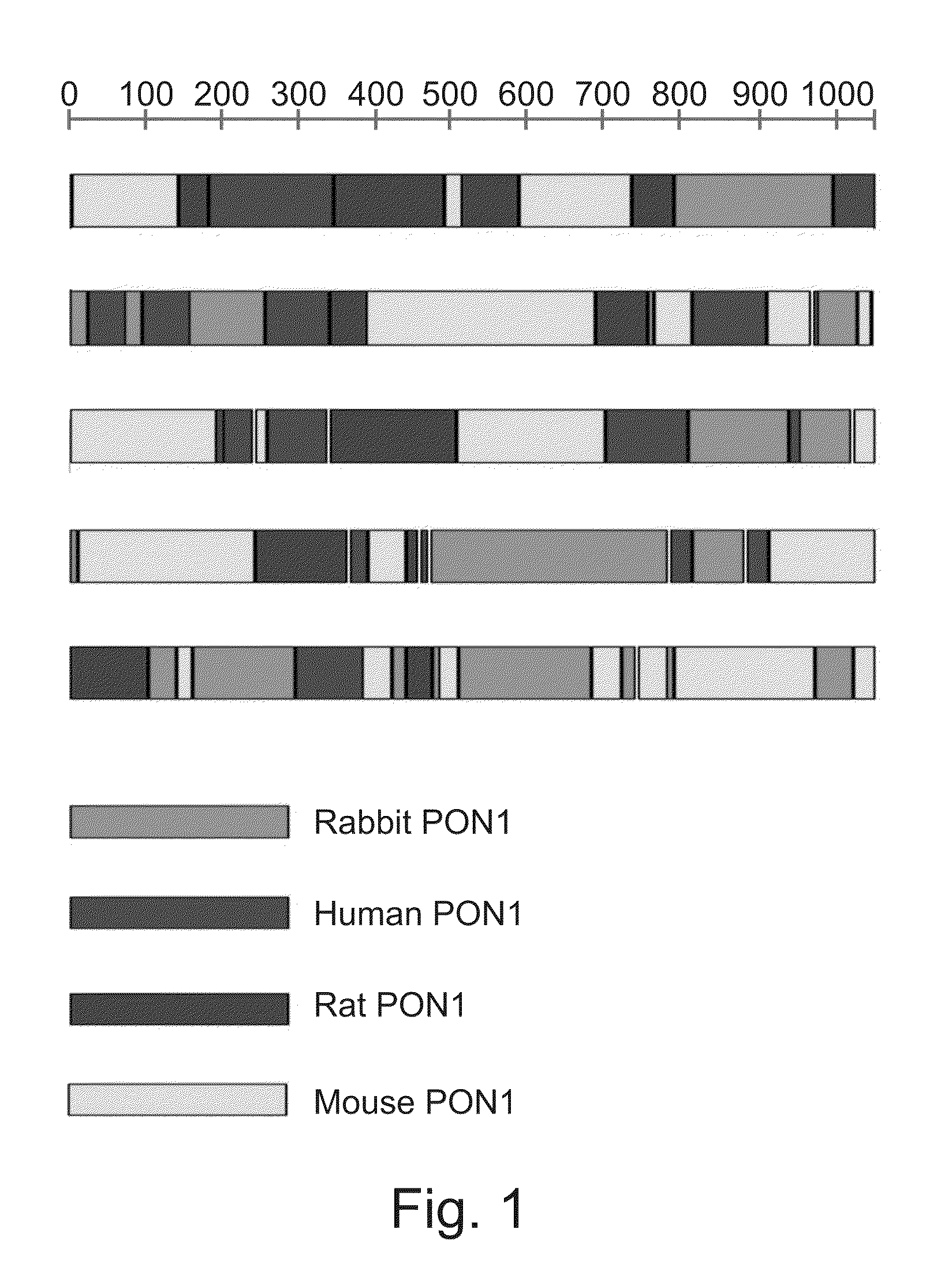
[0273]Family DNA shuffling—The PON1 genes, hPON1, mPON1, RatPON1 and RabPON1 were shuffled using established protocols (Abecassis et al., 2000; Crameri et al., 1998; Stemmer, 1994). A forward primer (T7-term-Fo, Table 1, above) and a reverse primer (pET32-Seq-bc, Table 1, above) were used to individually amplify the various PON1 genes from the respective pET32b(+) plasmid, using ExTaq (Takara). Equal amounts of the four DNA fragments were purified, mixed together and subjected to DNase I digestion (Bovine pancrease, Sigma). A 50 μl digestion contained ˜10 μg of DNA and 0.1 unit of DnaseI in 0.1 M Tris-HCl (pH 7.5) containing 10 mM manganese chloride. ...
example 3
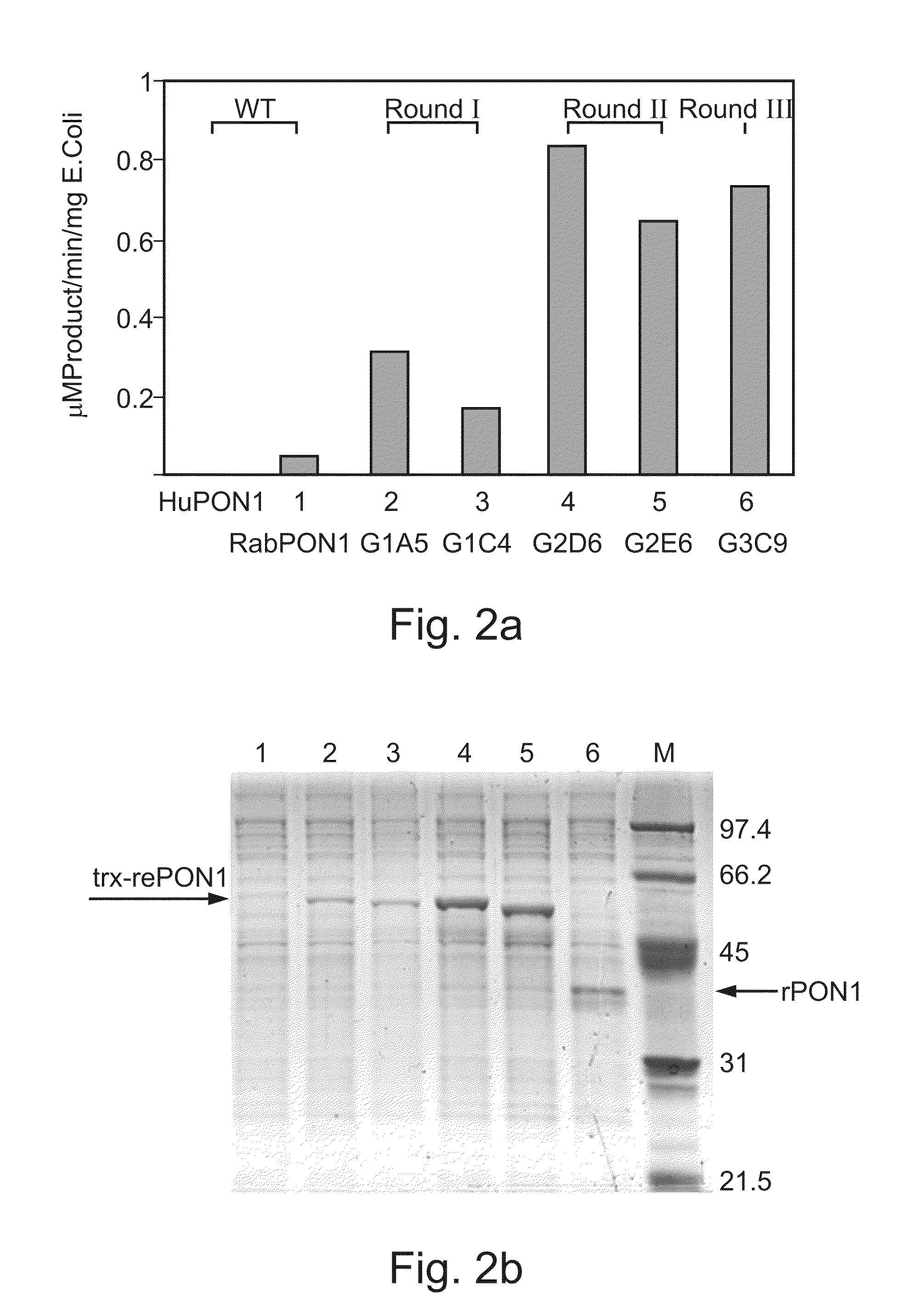
Characterization of Directly Evolved PON1 Variants
[0285]Sequence analysis of the directly evolved PON1 variants generated as described above was effected as is described herein below.
[0286]Materials and Experimental Procedures
[0287]Expression and purification of the directly-evolved PON1 variants. Origam B (DE3) cells were transformed with plasmid DNA isolated from selected variants. Single colonies were used to inoculate 5 ml of LB media supplemented with Amp (100 μg / ml), Kanamycin (15 μg / ml) and Calcium chloride (1 mM) and the resulting cultures were grown 0 / N at 30° C. Cells were harvested by centrifugation and resuspended in 60 ml of activity buffer supplemented with 1 μl of Pepstatin A, 0.1 mM DTT and 0.03% of n-dodecyl-β-D-maltopyranoside (C12-maltoside). The cells were disrupted by sonication and the suspension was gently shaken at room temperature for 2 hours. Cell debris was removed by centrifugation and ammonium sulfate was added to 50% saturation (w / v). The resulting prec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com