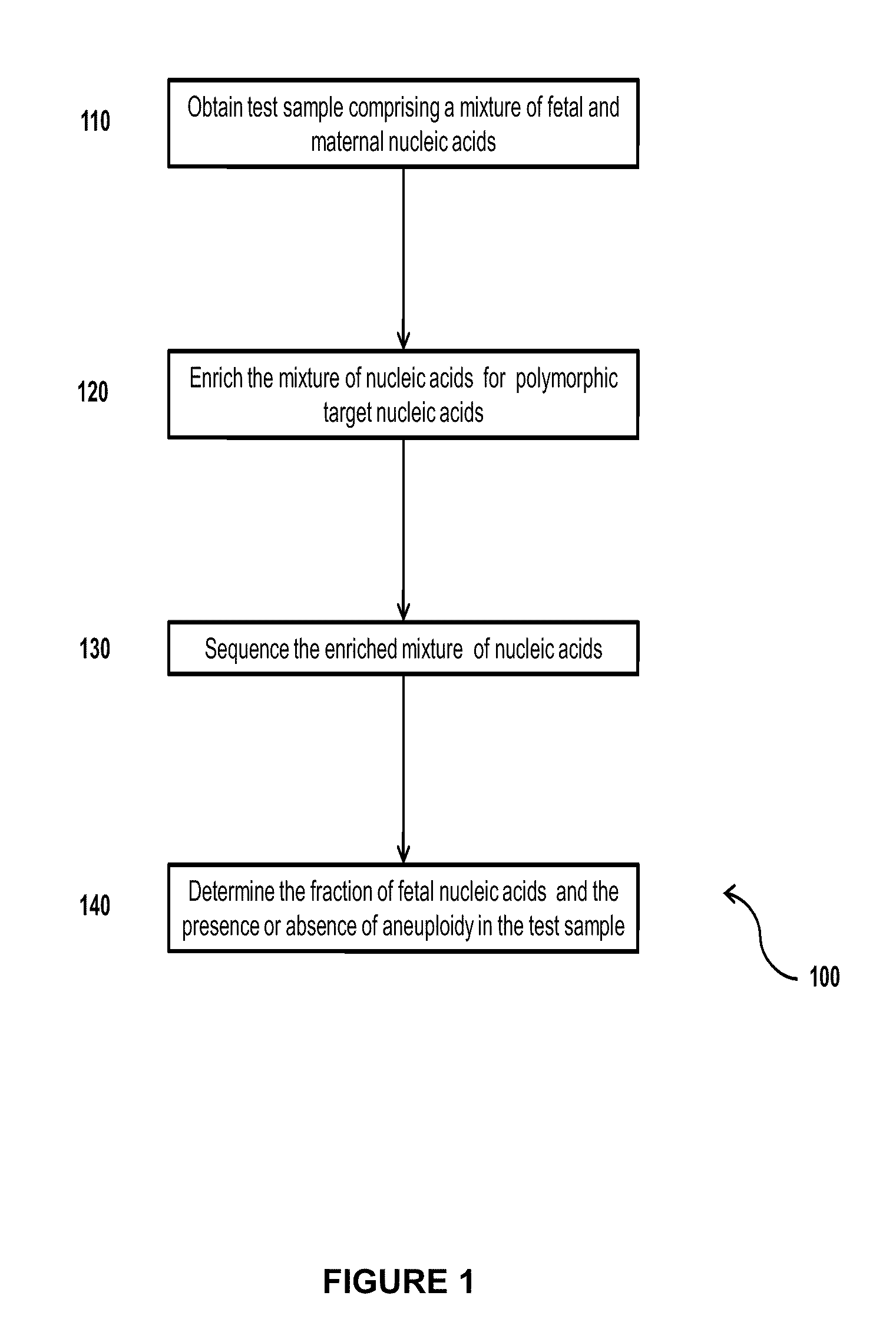
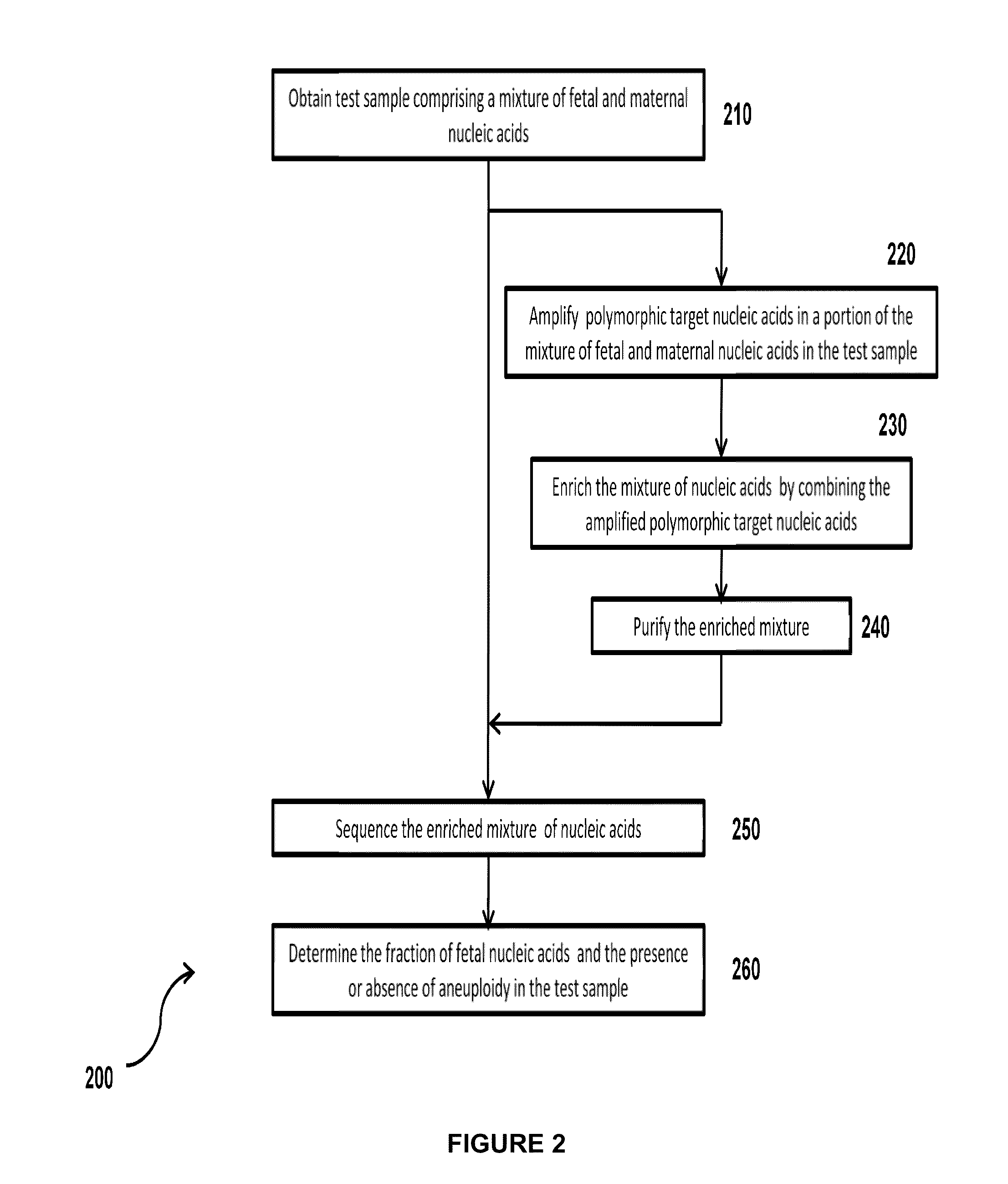
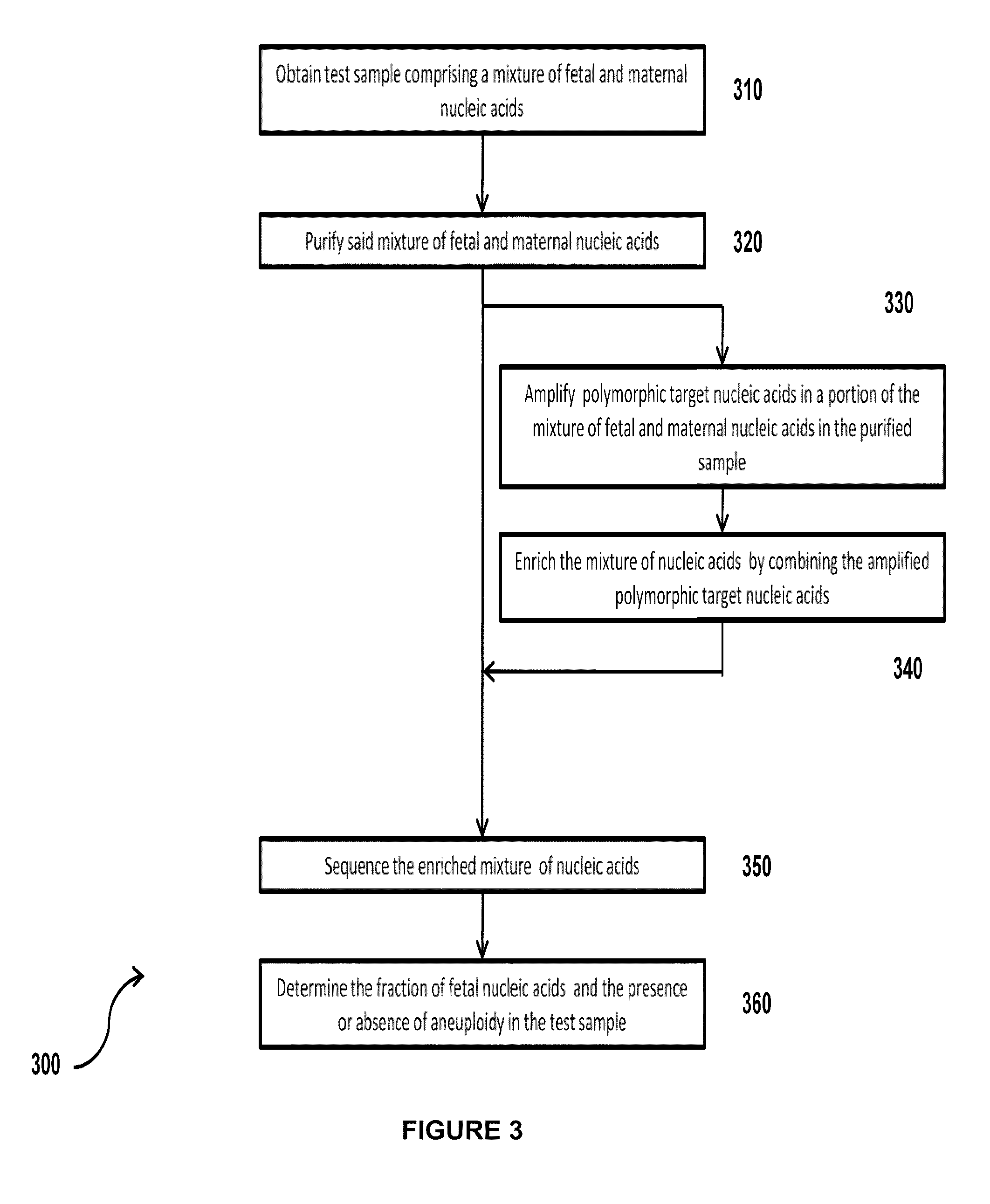
Simultaneous determination of aneuploidy and fetal fraction
a technology of fetal fraction and simultaneous determination, applied in the field of diagnostics, can solve the problems of false negative results and a great risk to the pregnancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Sample Processing and cfDNA Extraction
[0163]Peripheral blood samples were collected from pregnant women in their first or second trimester of pregnancy and who were deemed at risk for fetal aneuploidy. Informed consent was obtained from each participant prior to the blood draw. Blood was collected before amniocentesis or chorionic villus sampling. Karyotype analysis was performed using the chorionic villus or amniocentesis samples to confirm fetal karyotype.
[0164]Peripheral blood drawn from each subject was collected in ACD tubes. One tube of blood sample (approximately 6-9 mL / tube) was transferred into one 15-mL low speed centrifuge tube. Blood was centrifuged at 2640 rpm, 4° C. for 10 min using Beckman Allegra 6 R centrifuge and rotor model GA 3.8.
[0165]For cell-free plasma extraction, the upper plasma layer was transferred to a 15-ml high speed centrifuge tube and centrifuged at 16000×g, 4° C. for 10 min using Beckman Coulter Avanti J-E centrifuge, and JA-14 rotor. The two centri...
example 2
Preparation and Sequencing of Primary and Enriched Sequencing Libraries
[0167]a. Preparation of Sequencing Libraries
[0168]All sequencing libraries i.e. primary and enriched libraries, were prepared from approximately 2 ng of purified cfDNA that was extracted from maternal plasma. Library preparation was performed using reagents of the NEBNext™ DNA Sample Prep DNA Reagent Set 1 (Part No. E6000L; New England Biolabs, Ipswich, Mass.), for Illumina® as follows. Because cell-free plasma DNA is fragmented in nature, no further fragmentation by nebulization or sonication was done on the plasma DNA samples. The overhangs of approximately 2 ng purified cfDNA fragments contained in 40 μl were converted into phosphorylated blunt ends according to the NEBNext® End Repair Module by incubating in a 1.5 ml microfuge tube the cfDNA with 5 μl 10× phosphorylation buffer, 2 μl deoxynucleotide solution mix (10 mM each dNTP), 1 μl of a 1:5 dilution of DNA Polymerase I, 1 μl T4 DNA Polymerase and 1 μl T4 ...
example 3
Analysis of Sequencing Data for the Determination of Aneuploidy and Fetal Fraction
[0170]a. Analysis of Sequencing Data for the Determination of Aneuploidy
[0171]Upon completion of sequencing of the sample, the Illumina “Sequencer Control Software” transferred image and base call files to a Unix server running the Illumina “Genome Analyzer Pipeline” software version 1.51. The Illumina “Gerald” program was run to align sequences i.e. 36 bp reads, to the hg18 reference human genome provided by National Center for Biotechnology Information (NCBI36 / hg18, available on the world wide web at genome.ucsc.edu / cgi-bin / hgGateway?org=Human&db=hg18&hgsid=166260105). The sequence data generated from the above procedure that uniquely aligned to the genome was read from Gerald output (export.txt files) by a program (c2c.p1) running on a computer running the Linux operating system. Sequence alignments with base mis-matches were allowed and included in alignment counts only if they aligned uniquely to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| polymorphic | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com