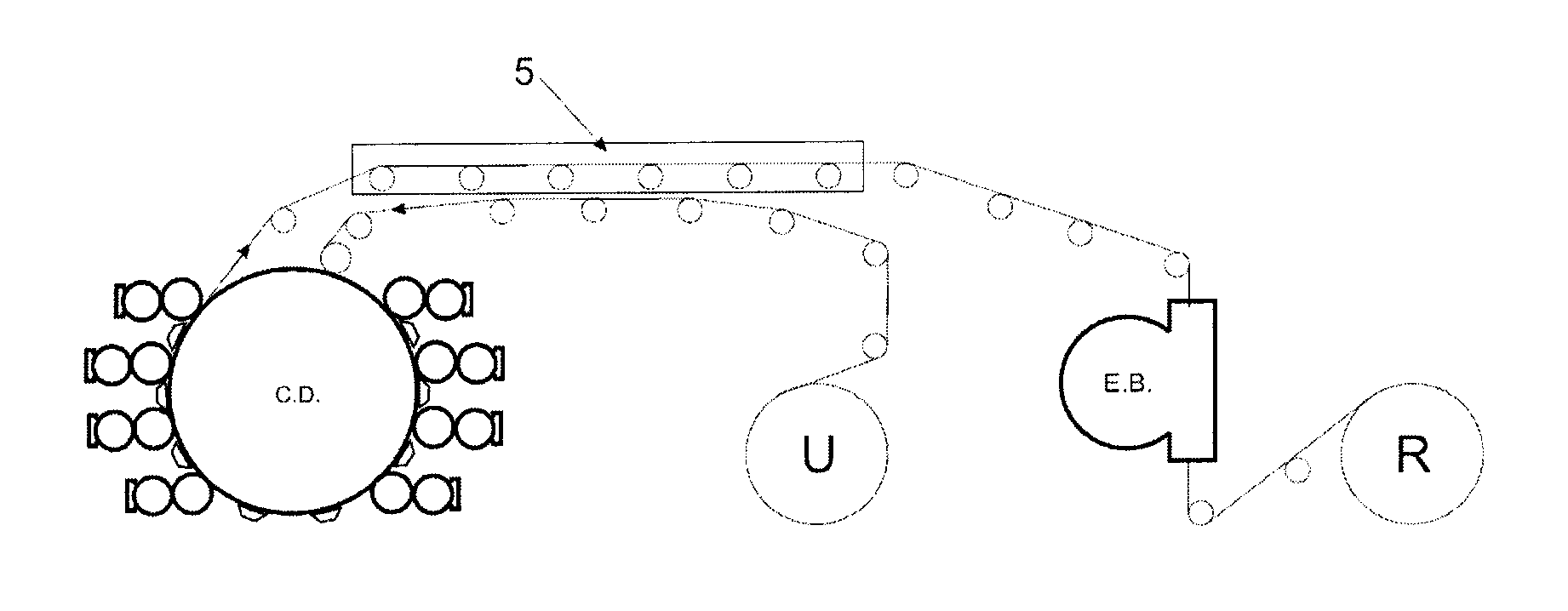
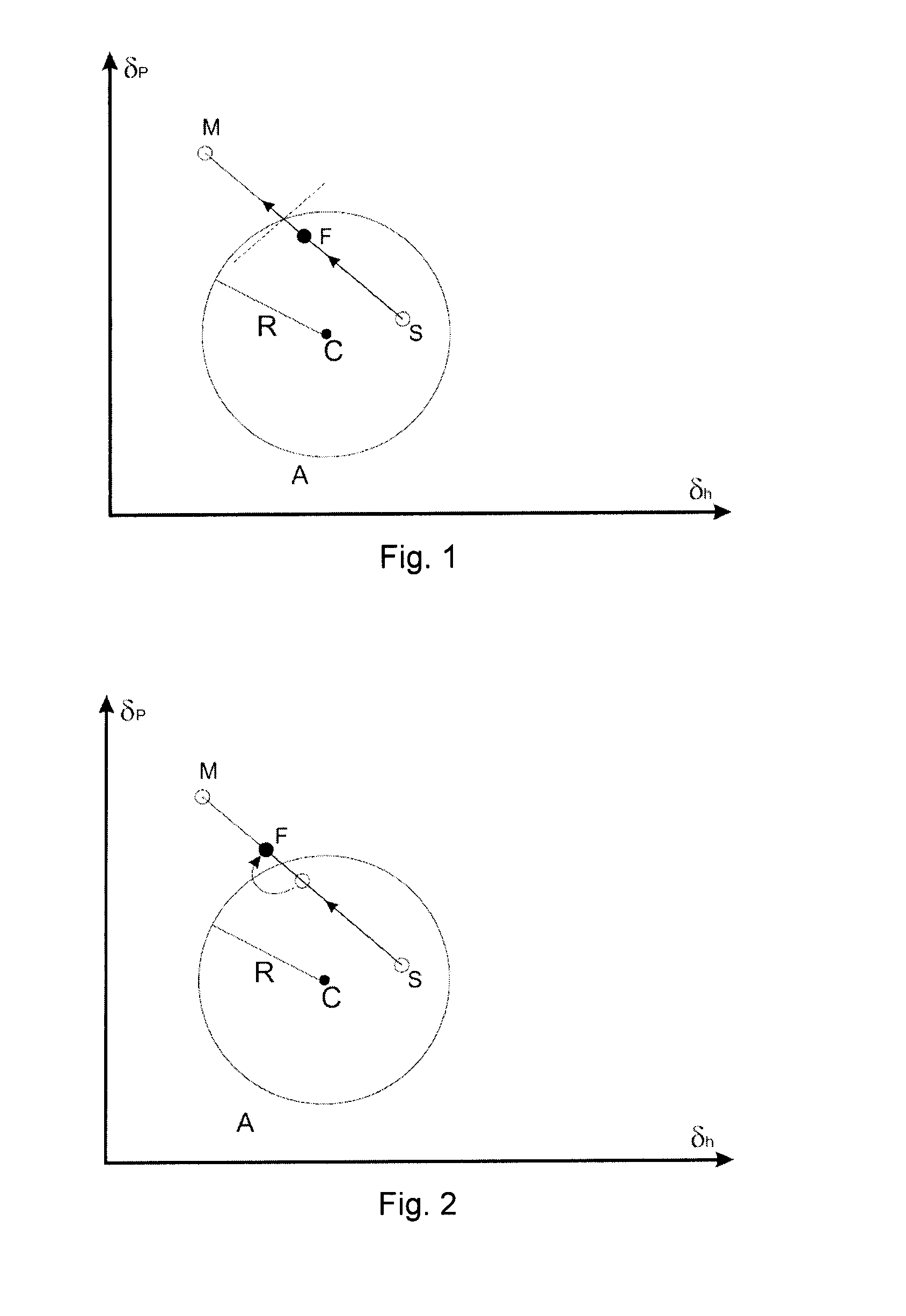
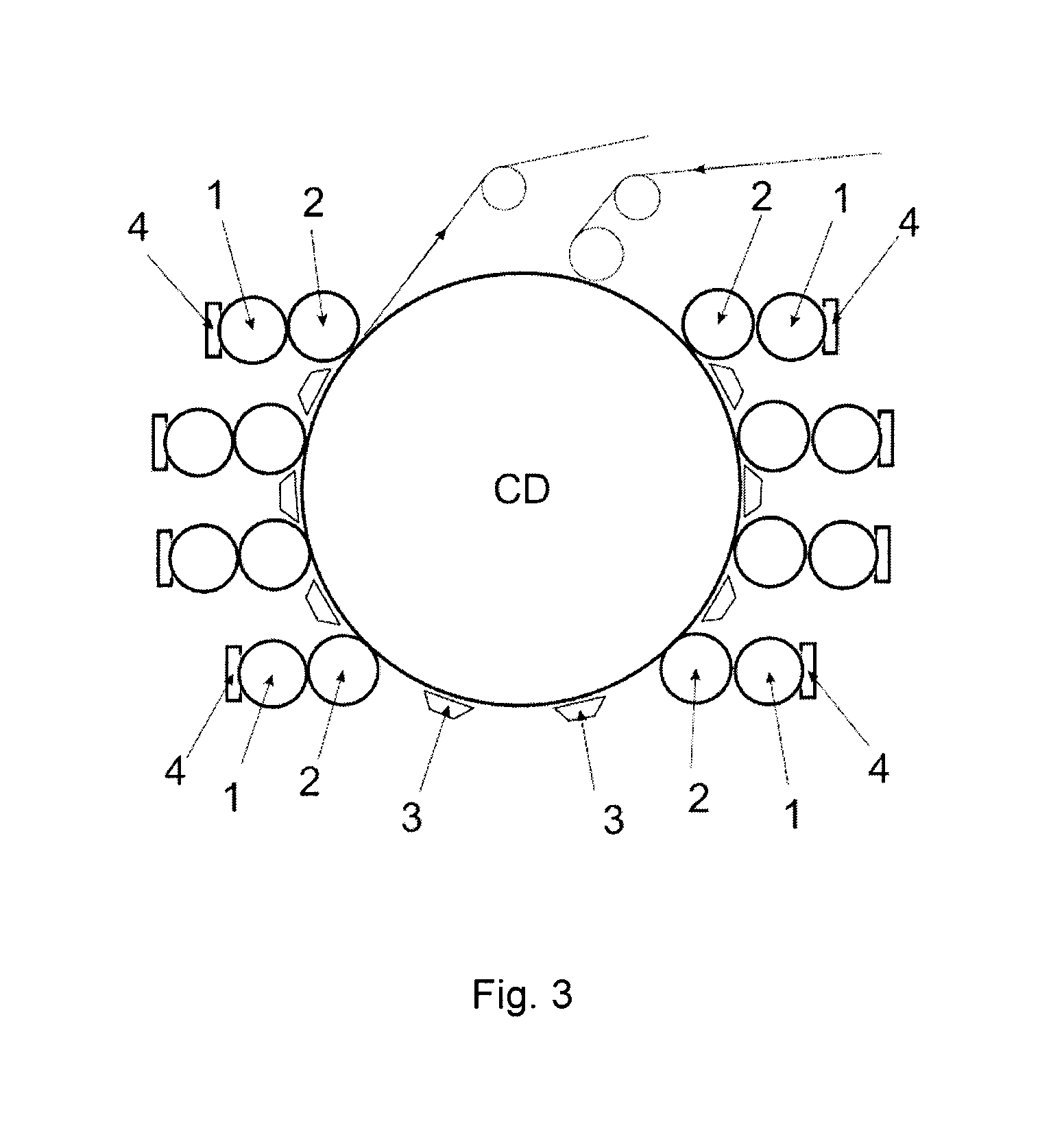
Flexographic printing process with wet on wet capability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Ink with Small Amount of Solvent
[0184]Formula A is for an EB curable ink formulated according to the present invention for a non-food application that contains only 5% solvent and 0.5% of Polymer (Polyvinyl Butyral-Butvar® B76): Formula A is showed on Table 4 below.
TABLE 4ink with small amount of solventFORMULA AProductTrade NameSupplierYellowMagentaCyanBlackAdditiveOmnistab 510IGM Resins0.50.50.50.5AdditiveTego Glide 432Tego Chemie1.01.01.01.0AdditiveTego Dispers 685Tego Chemie4.02.5——AdditiveDisperbyk 168Altana3.05.56.55.0MonomerTMPTACytec51.051.051.048.5MonomerHDDACytec5.05.05.04.5Epoxidized SoyCN111Sartomer5.56.06.06.0Bean Oil AcrylateAdditiveSolsperse 22000Noveon2.0———AdditiveSolsperse 5000Noveon——1.52.0Yellow PigmentIrgalite Yellow LCTCIBA22.5———Magenta PigmentPermanent Rubine L4B 01Clariant—23.0——Cyan PigmentHeliogen LBL 7081DBASF——23.0—Black PigmentSpecial Black 250Degussa———27.0PolymerButvar B76Solutia0.50.50.50.5SolventDowanol PMDow5.05.05.05.0Viscosity (cps)15001750135021...
example 2
Ink with Increased Amount of Polymer
[0203]In example 2, the amount of polymer was increased, that means a elevation in the polymer network density and in the amount of change in the Hansen Solubility Parameter, in order to ensure a sufficient distance between the liquid and gel state, avoiding ink gelation in the inking system. Table 7 shows Formula B.
TABLE 7FORMULA BProductTrade NameSupplierQuantityAdditiveOmnistab 510IGM Resins0.50.50.50.5AdditiveTego Glide 432Tego Chemie1.01.01.01.0AdditiveTego Dispers 685Tego Chemie4.02.5——AdditiveDisperbyk 168Altana3.05.56.55.0MonomerTMPTACytec46.546.546.544.0MonomerHDDACytec3.03.03.02.5Epoxidized SoyCN111Sartomer5.56.06.06.0Bean Oil AcrylateAdditiveSolsperse 22000Noveon2.0———AdditiveSolsperse 5000Noveon——1.52.0Yellow PigmentIrgalite Yellow LCTCIBA22.5———Magenta PigmentPermanent Rubine L4B 01Clariant—23.0——Cyan PigmentHeliogen LBL 7081DBASF——23.0—Black PigmentSpecial Black 250Degussa———27.0PolymerButvar B76Solutia2.02.02.02.0SolventDowanol PMDo...
example 3
Composition Approved for Food Packing
[0212]Using the same general formulation principle, but using only FDA approved monomer for Food Packing, the previous formulations is changed by exchanging HDDA with TRPGDA (Tripropylene Glycol Diacrylate).
[0213]In both case, the use of HDDA and / or TRPGDA is directed to bring the TMPTA as close as possible to the solubility border of Butvar® B76 in order to reduce as much as possible the Methoxy Propanol (Dowanol PM) level in the ink, since after its evaporation there is an adverse effect of VOLATILE ORGANIC COMPOUNDS in the atmosphere. Table 10 below shows formula C:
TABLE 10gelled ink with small amount of solventFORMULA CProductTrade NameSupplierQuantityAdditiveOmnistab 510IGM Resins0.50.50.50.5AdditiveTego Glide 432Tego Chemie1.01.01.01.0AdditiveTego Dispers 685Tego Chemie4.02.5——AdditiveDisperbyk 168Altana3.05.56.55.0MonomerTMPTACytec37.537.537.536.5MonomerTRPGDACytec12.012.012.011.0Epoxidized SoyCN111Sartomer5.56.06.06.0Bean Oil AcrylateAddi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com