Biodegradation of renewable hydrocarbon fuel blends
a technology of renewable hydrocarbon fuel and biodegradation, which is applied in the direction of biofuels, liquid carbonaceous fuels, fuels, etc., can solve the problems of enhanced btex plumes and lag in btex degradation, and achieve the effect of improving the environmental fate of hydrocarbon fuel compositions and increasing the renewability of said fuel compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Aerobic Testing
Materials and Methods
[0056]Soil and Groundwater Samples
[0057]Soil and groundwater for laboratory microcosm testing were collected from within Site 60 at Vandenberg Air Force Base, CA. The site has a history of gasoline contamination, but has undergone an extensive cleanup program. Collected groundwater was containerized in sterile stainless steel soda kegs (18.5 L) under nitrogen headspace. Soil located approximately 8 to 12 feet below ground surface (bgs) was collected using a Geoprobe® 6620DT with acetate core sleeves. The core samples in acetate sleeves were capped and sealed in the field to minimize exposure to air, shipped overnight on ice to the laboratory, and stored at 4° C.
[0058]Soil was removed from the acetate sleeves in an anaerobic chamber (Coy Laboratory Products, Inc., Grass Lake, Mich.) and the first 10 cm of the core ends that may have been exposed to oxygen were discarded. Collected soil consisted of silty sand with some gravel and larger stones. The...
example 2
Anaerobic Testing
Materials and Methods
Soil and Groundwater
[0072]The soil and groundwater and sample collection and handling procedures are as described above. Baseline soil and groundwater data are presented in Table 4. (NA=Not Analyzed; * SVOC detections include 0.008 mg L-1 phenol and 0.003 mg L-1 bis(2-ethylhexyl) phthalate; ** standard units)
TABLE 4Groundwater and soil parametersParameterGroundwater (mg / L)Soil (mg / kg)Total Organic Carbon 221,700Gasoline Range 5.7NAOrganicsTotal SVOCs 0.011* 0.084Total Iron490NADissolved Iron NANitrate (as N) 1.5NASulfate (as SO42−)105NAAlkalinity (as CaCo3)391NAMethane 0.005NApH 7.2**NADissolved Oxygen 0.8NA
Microcosm Preparation
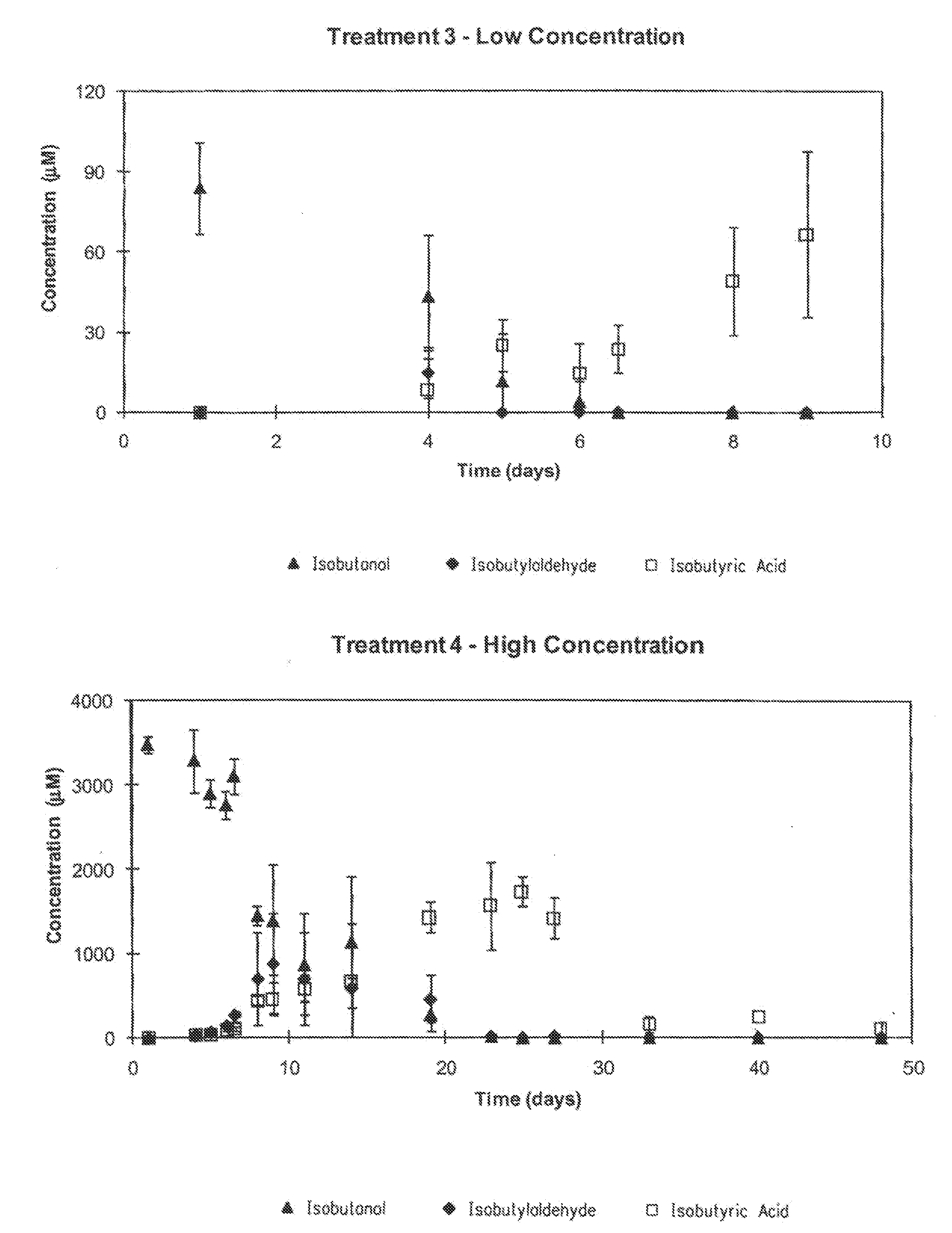
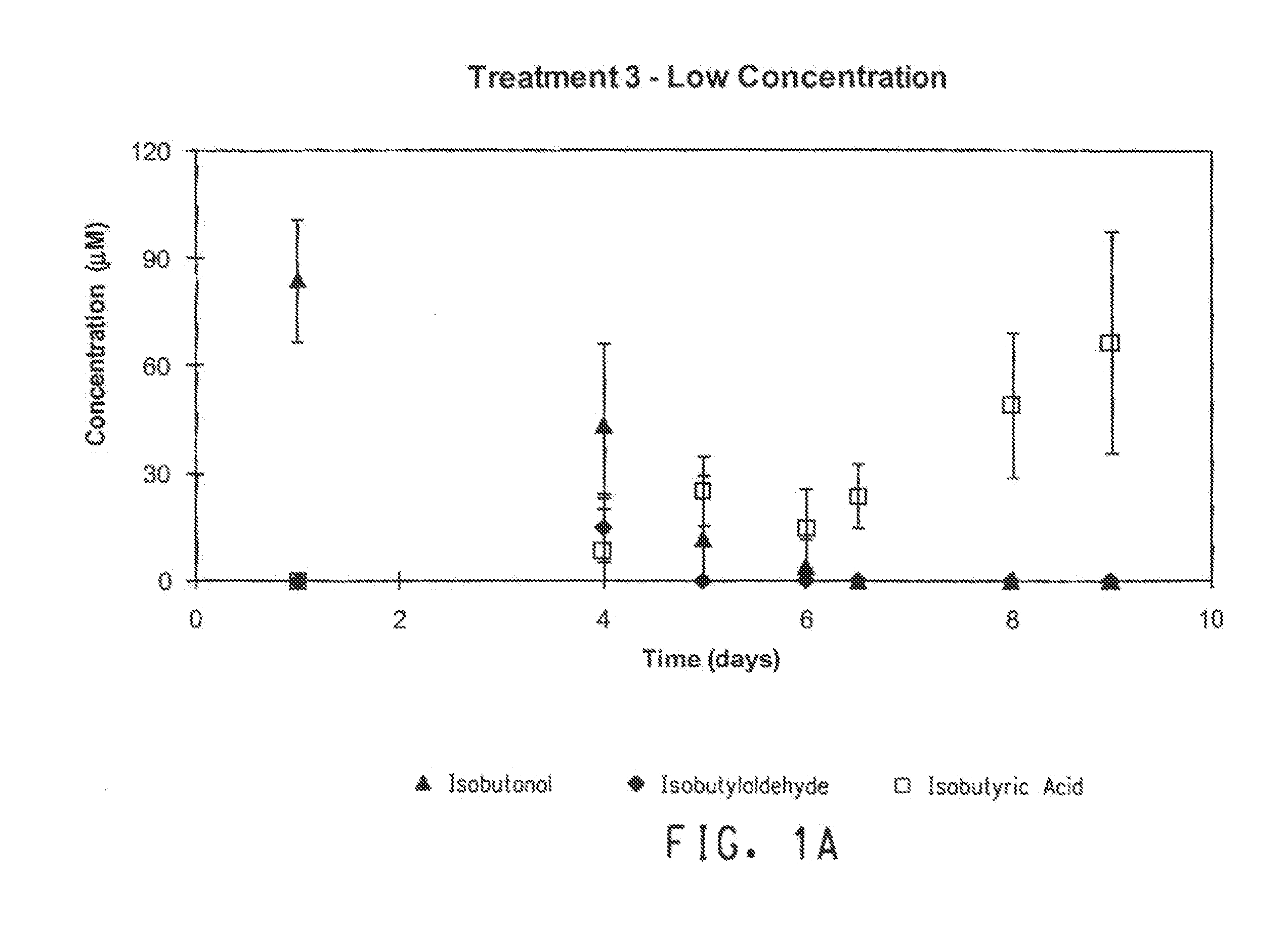
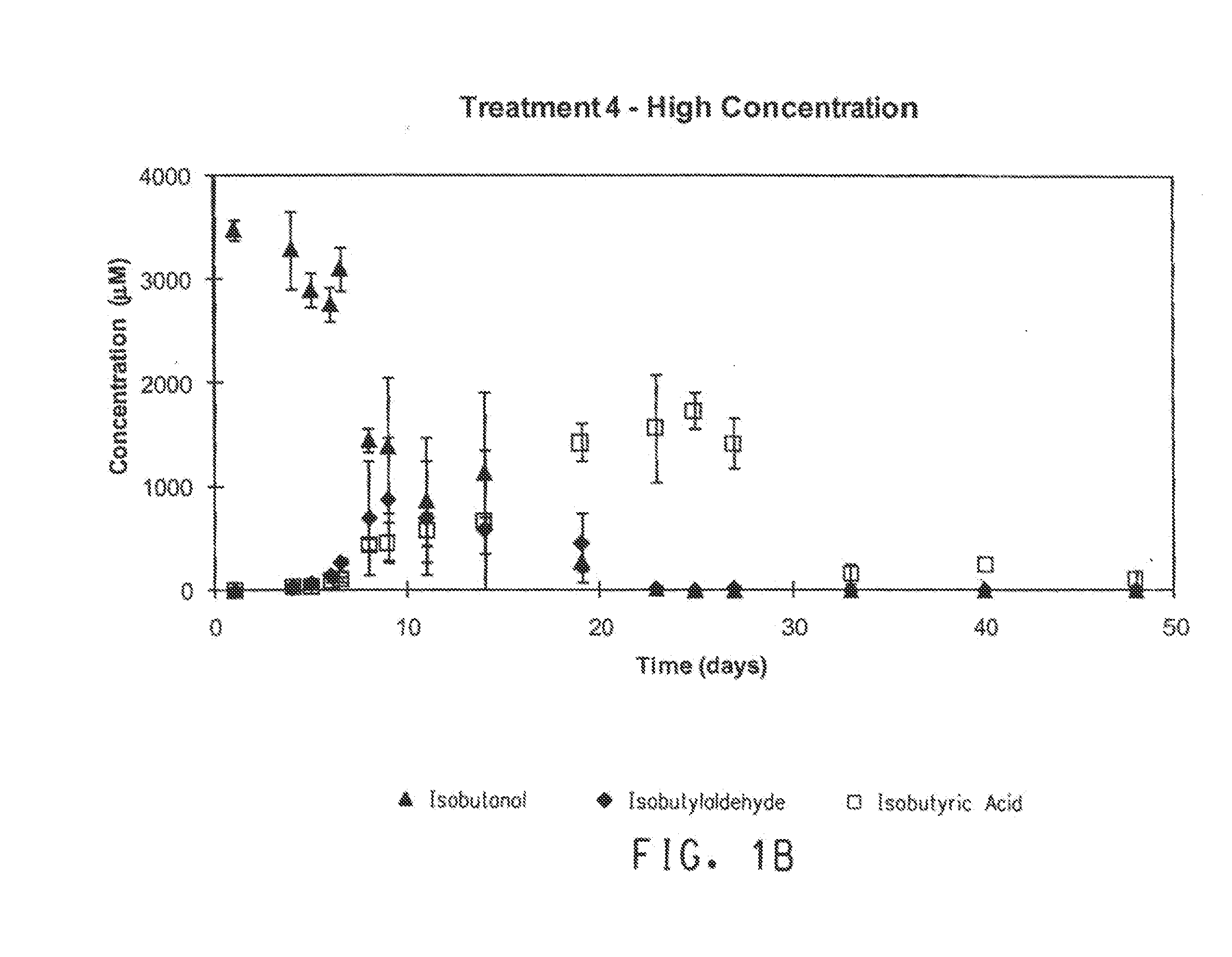
[0073]For the microcosm experiments, the biodegradation of BTEX and isobutanol in soil-groundwater slurries under conditions ranging from nitrate-reducing to methanogenic were evaluated. BTEX and isobutanol were evaluated at “high” and “low” concentrations (Table 5). Two treatments were prepared using ethanol instead of ...
example 3
Isobutanol as a Co-Solvent for Ethanol
[0092]Scoping water tolerance and phase separation tests were conducted on isobutanol-ethanol-gasoline blends at 65° F. Increasing amounts of isobutanol were mixed with E10 gasoline and then either 1.3% or 2.6% water were added to all of the blends. The water needed to be increased to 2.6 vol % for some blends because gasoline blends containing ethanol absorb larger amounts of water and 1.3% water was not always sufficient to induce the formation of separate aqueous and hydrocarbon phases for analysis (i.e., the 1.3 vol % water was completely absorbed by the higher isobutanol blends). This can be seen in FIG. 11 where 1.3 vol % was sufficient to cause two phases until the amount of isobutanol in E10 reached 5 vol %, but for 10 vol % isobutanol in E10, the level needed to be increased to 2.6 vol % water to induce phase separation.
[0093]The data in FIG. 12 show that the amount of ethanol extracted into the aqueous phase decreased with increasing i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| octanol-water partition coefficient | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com