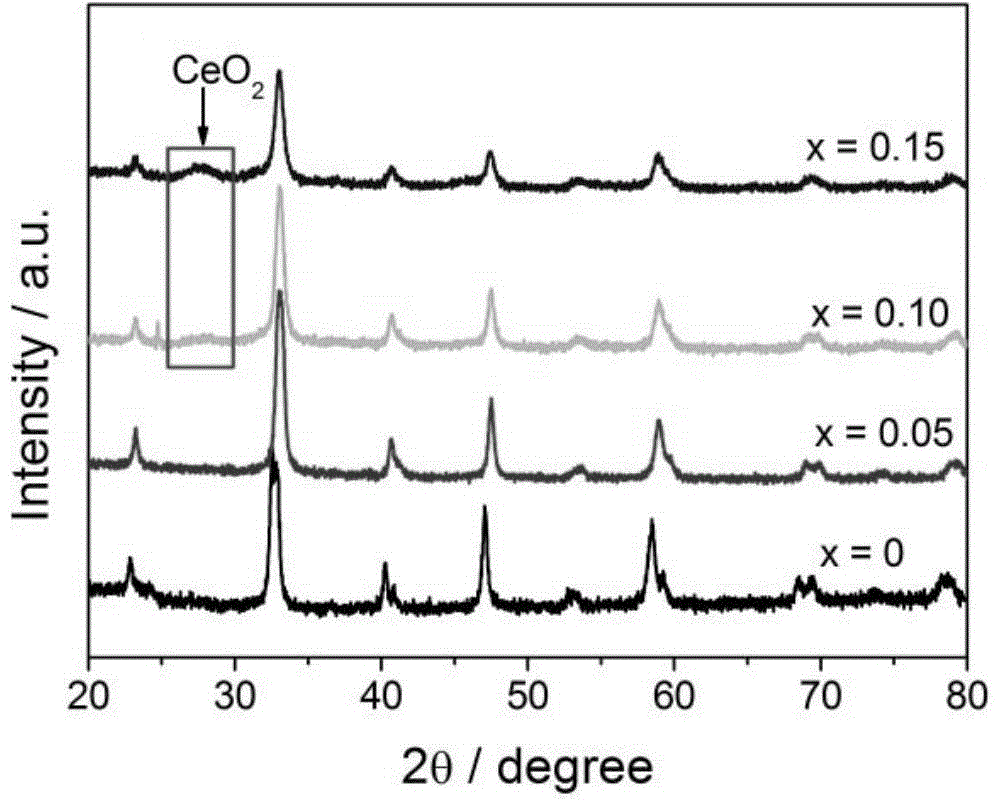
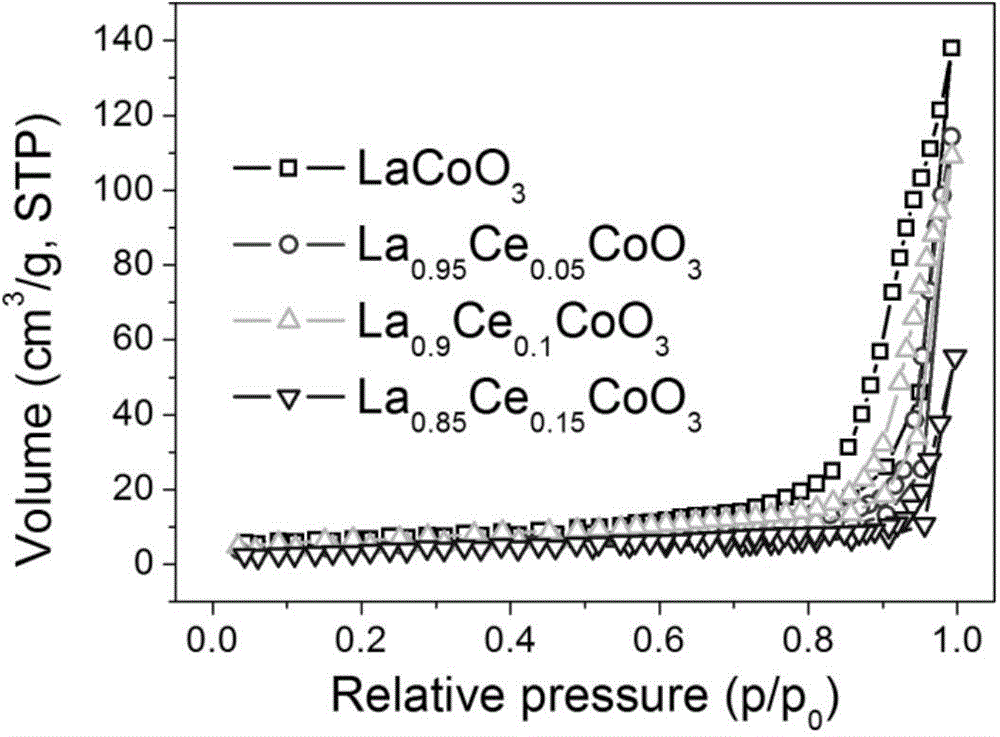
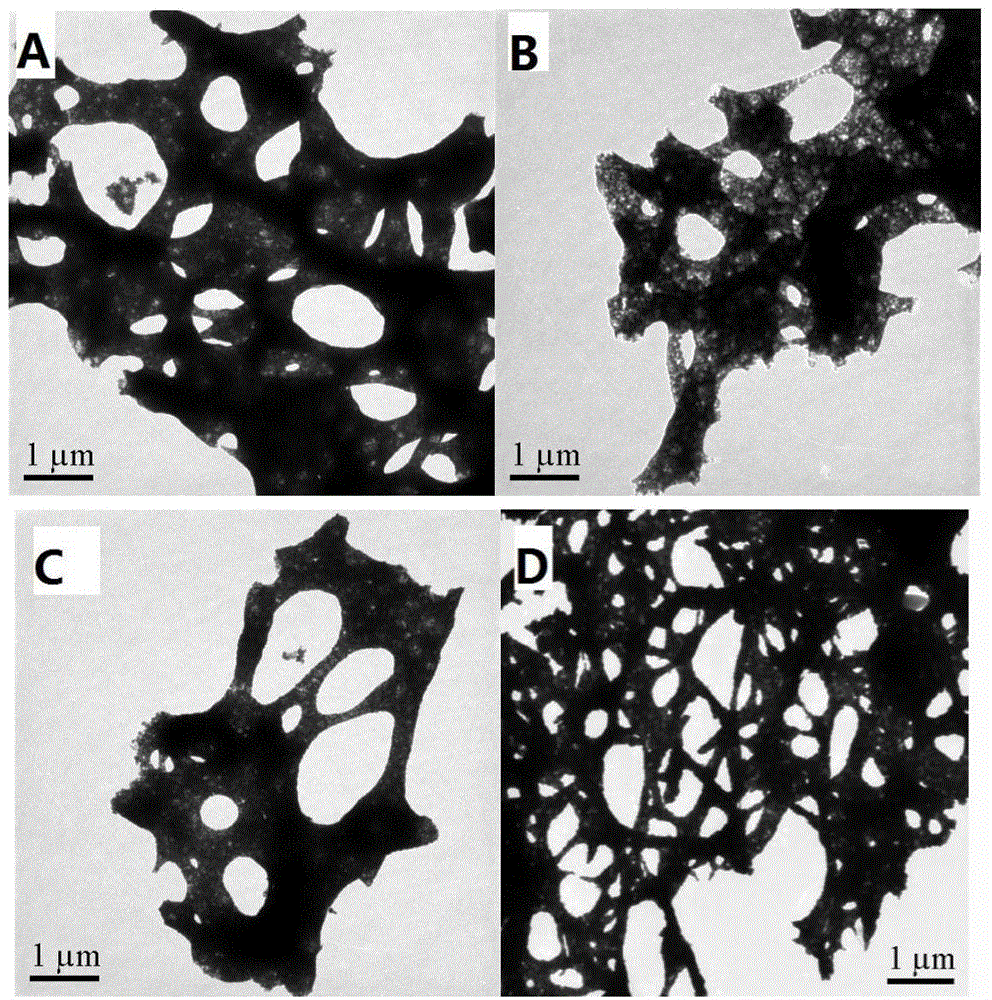
Preparation method and application of porous La1-xCexCoO3 perovskite catalyst
A catalyst and mixed solvent technology, applied in the preparation and application of new materials, can solve the problems of reduced catalyst selectivity and high price, and achieve the effects of good catalytic oxidation activity and stability, low price and good catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A La 1-x Ce x CoO 3 The catalyst is prepared by a sol-gel method, and its specific preparation steps are as follows:
[0035] (1) The lanthanum nitrate and cerium nitrate mixture, 5mmol cobalt nitrate that the total molar number is 5mmol are joined in the mixed solvent that is made up of 3mL ethylene glycol and 2mL methanol, stir and dissolve;
[0036] (2) drying the reaction mixture obtained in step (1) in an oven at 95°C to obtain a gel-like solid;
[0037] (3) After taking out the gelatinous solid obtained in step (2), place it in a muffle furnace for calcining and crystallization to obtain the target product, and take it out to obtain La 1-x Ce x CoO 3 catalyst.
[0038] The process of calcination and crystallization in the muffle furnace is as follows: the temperature is raised from room temperature to 600° C. at a rate of 1° C. / min, kept for 5 hours, and then naturally cooled to room temperature.
[0039] Said x is the mole fraction of cerium nitrate in the...
Embodiment 2
[0049] Four kinds of La that embodiment 1 prepares respectively 1-x Ce x CoO 3 (x=0, 0.05, 0.1, 0.15) the catalytic performance of the catalyst in the catalytic oxygen oxidation of benzyl alcohol to benzaldehyde is tested:
[0050] The experimental process is as follows: Take 20 mL of toluene, 20 μL of benzyl alcohol, 10 μL of dodecane (as an internal standard), 0.05 g of catalyst, mix and stir in a three-necked flask, and pass O 2 (99.99%), the flow rate was 50 mL / min, and the reaction temperature was maintained at 88°C. The product was analyzed by gas chromatography, and the detector was flame ionization detector, separation column HP-5 capillary column, H 2 For the carrier gas. The conversion rate of benzyl alcohol is as attached Figure 6 As shown in A. It can be seen from the figure that the addition of a small amount of Ce improves the activity of the reaction and reaches the maximum when x=0.1, indicating that the incorporation of Ce in the perovskite lattice help...
Embodiment 3
[0052] To illustrate the necessity of the presence of a catalyst for the reaction, we examine the La 0.9 Ce 0.1 CoO 3 Catalyst before and after filtration to the catalytic activity of benzyl alcohol oxidation to benzaldehyde reaction as a function of time, see the attached Figure 6 b. Reaction conditions and experimental process are with embodiment 2. It can be seen that when the catalyst exists, the reaction activity gradually increases with the prolongation of time, and when the catalyst is removed, the reaction activity basically does not change, indicating that the reaction is not a homogeneous reaction, but mainly depends on La 0.9 Ce 0.1 CoO 3 Catalytic ability of the catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com