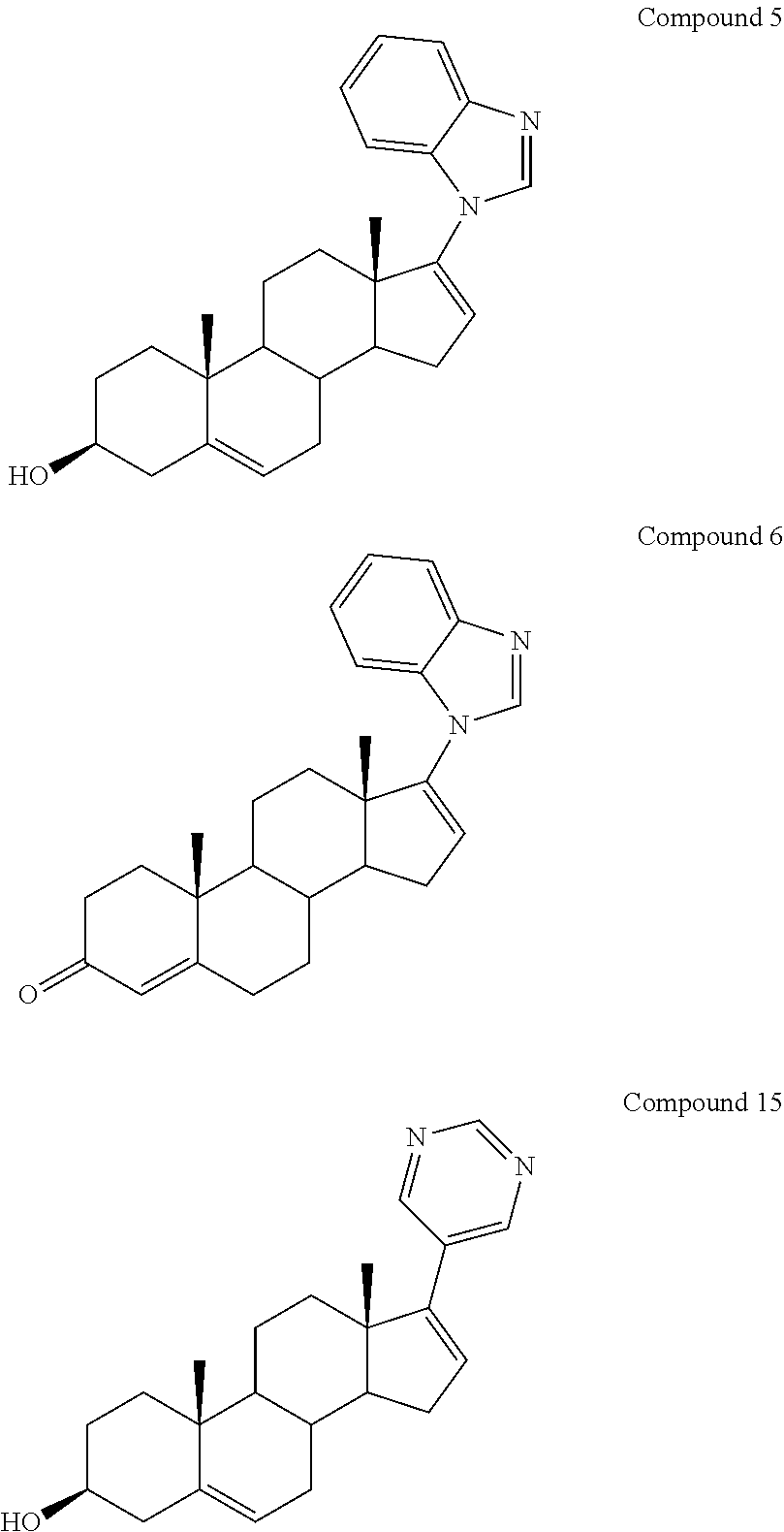
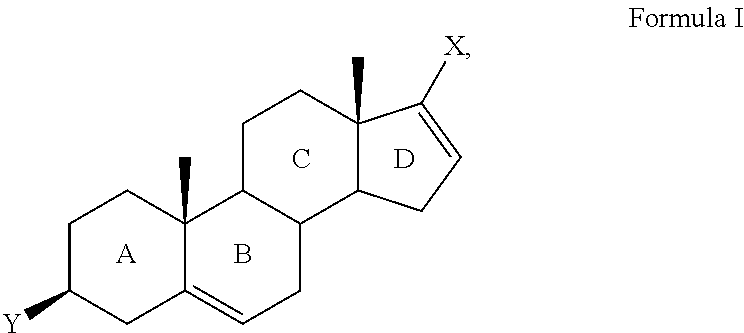
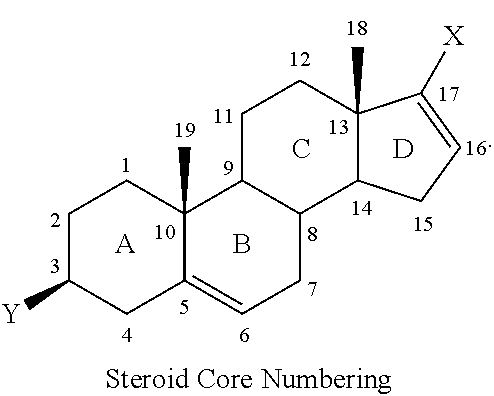
Novel prodrugs of steroidal cyp17 inhibitors/antiandrogens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Betaine Ester of Abiraterone
[0123]
[0124]A solution of bromoacetic acid (3.0 mmol 417 mg) in dichloromethane (10 mL) is stirred while dicyclohexylcarbodiimide (3.0 mmol, 619 mg), dimethylaminopyridine (0.5 mmol, 61 mg), followed by a solution of abiraterone (2.9 mmol, 1.08 g) in dichloromethane (3 mL) are added. The resultant mixture is stirred at room temperature for four hours. The mixture is filtered to remove precipitated dicyclohexyl urea, and poured into ethyl acetate. The organic layers are washed (1N HCL, 5% sat'd NaHCO3), dried (brine, MgSO4), and concentrated, with purification by column chromatography affording the pure alpha-halo ester.
[0125]The above-prepared bromoester (1.5 mmol, 743 mg) is dissolved in acetone (10 mL) and triethylamine (2.5 mmol, 253 mg, 3504) is added. The mixture is stirred until the steroid starting material is shown to be exhausted by TLC. The reaction mixture is concentrated in vacuo, and the residue is purified by reversed-phase HPLC to afford pu...
example 2
Carnitine Ester of Abiraterone
[0127]
[0128]A solution of R-dimethylmalate (10 mmol, 1.62 g) in THF (40 mL) is cooled at −78° C. and stirred while borane-dimethylsulfide complex (9.5 mmol, 4.75 mL of a 2.0M solution) in THF is added. The mixture is allowed to warm to room temperature and stirred while heating at reflux until exhaustion of the starting diester is indicated by TLC. The reaction mixture is quenched by slow addition of THF-water (1:1, 10 mL), and the resulting mixture is carefully poured into a solution of sodium hydroxide (5M, 10 mL), and stirred overnight. The reaction mixture is concentrated in vacuo, and the residue is taken up into ethyl acetate (50 mL). The organic layer is washed (1N, HCl, 5% sat'd aq NaHCO3), dried (brine, MgSO4), and concentrated in vacuo, with the residue being distilled in vacuo to afford purified methyl R-3,4-dihydroxybutyrate, or the residue may be used directly in the following step.
[0129]A solution of methyl R-3,4-dihydroxybutryate (6 mmol,...
example 3
Gallic Acid Ester of Abiraterone
[0136]
[0137]A solution of abiraterone (2 mmol, 747 mg), 3,4,5-tris[(tert-butyl dimethylsilyl)oxy]benzoic acid (2 mmol, 1.026 g), and 4-dimethylaminopyridine (1.0 mmol, 122 mg) in dichloromethane (10 mL) is stirred while dicyclohexylcarbodiimide (2.0 mmol, 412 mg) is added. The resultant suspension is stirred for three hours, and then filtered to remove precipitated dicyclohexylurea. The filtrate is washed with 1N HCl (2×50 mL), and the acid layers are extracted with dichloromethane (1×100 mL). The combined organics are dried (brine, MgSO4), and concentrated in vacuo to afford a solid. The solid is purified by flash column chromatography (silica gel, CHCl3—MeOH) to afford the pure tris-silyl protected ester.
[0138]The above prepared ester is dissolved in THF (8 mL) and TBAF is added as a THF solution (1M, 6 mL, 6 mmol) and the resultant solution is stirred for two hours at room temperature. The mixture is poured into half-saturated aqueous sodium chlori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com