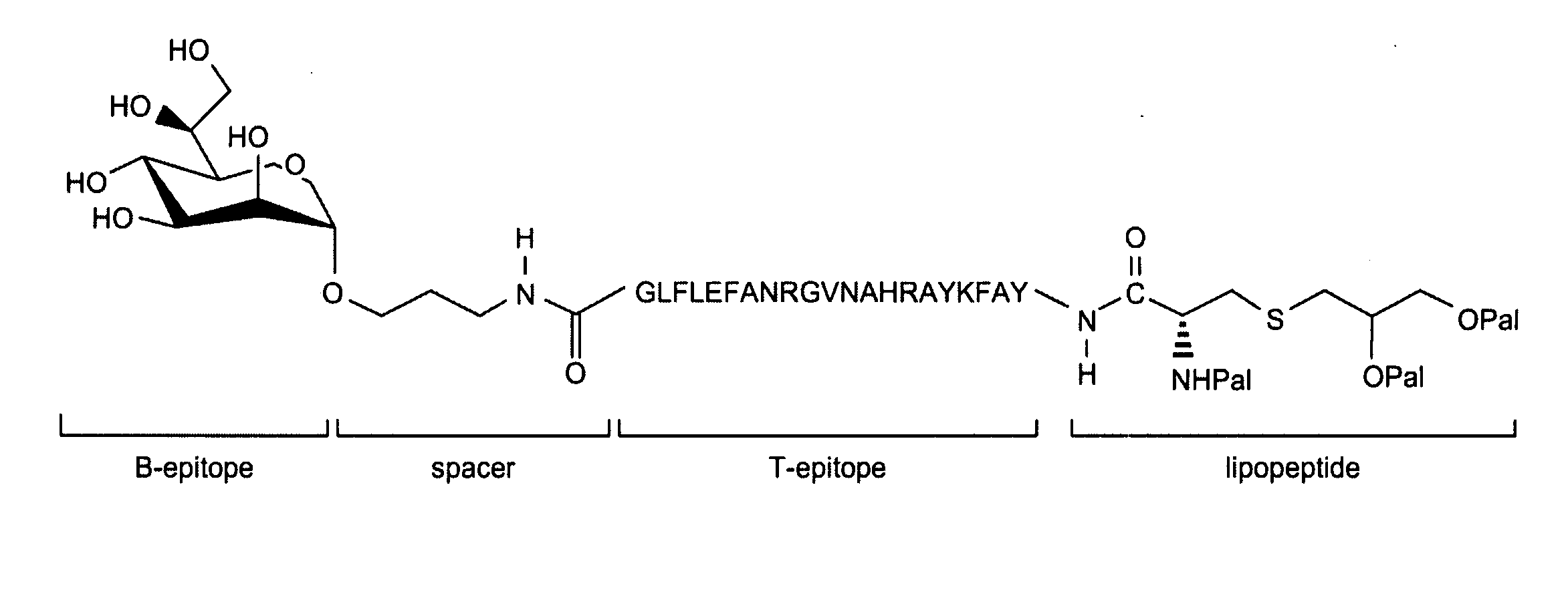
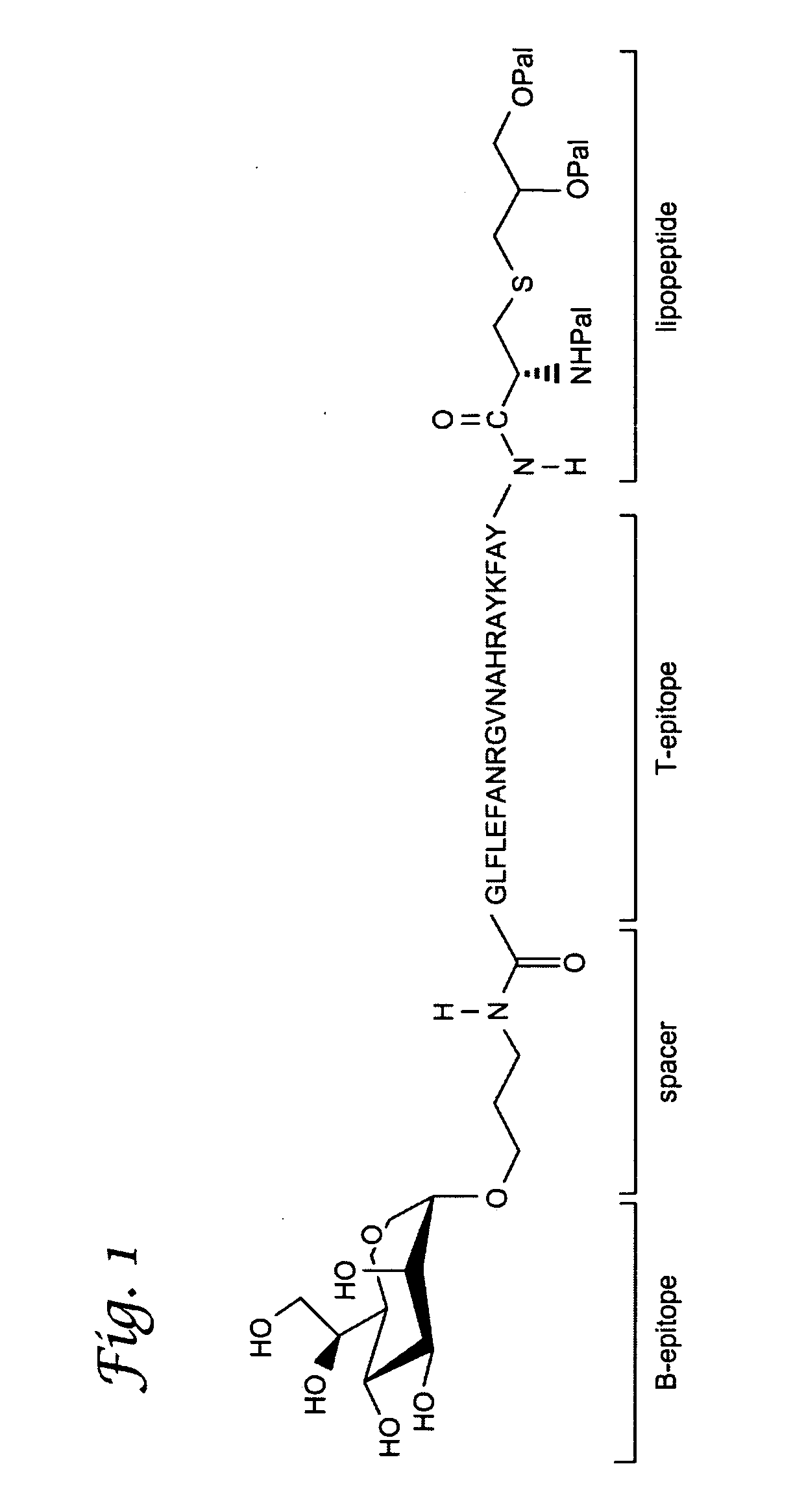
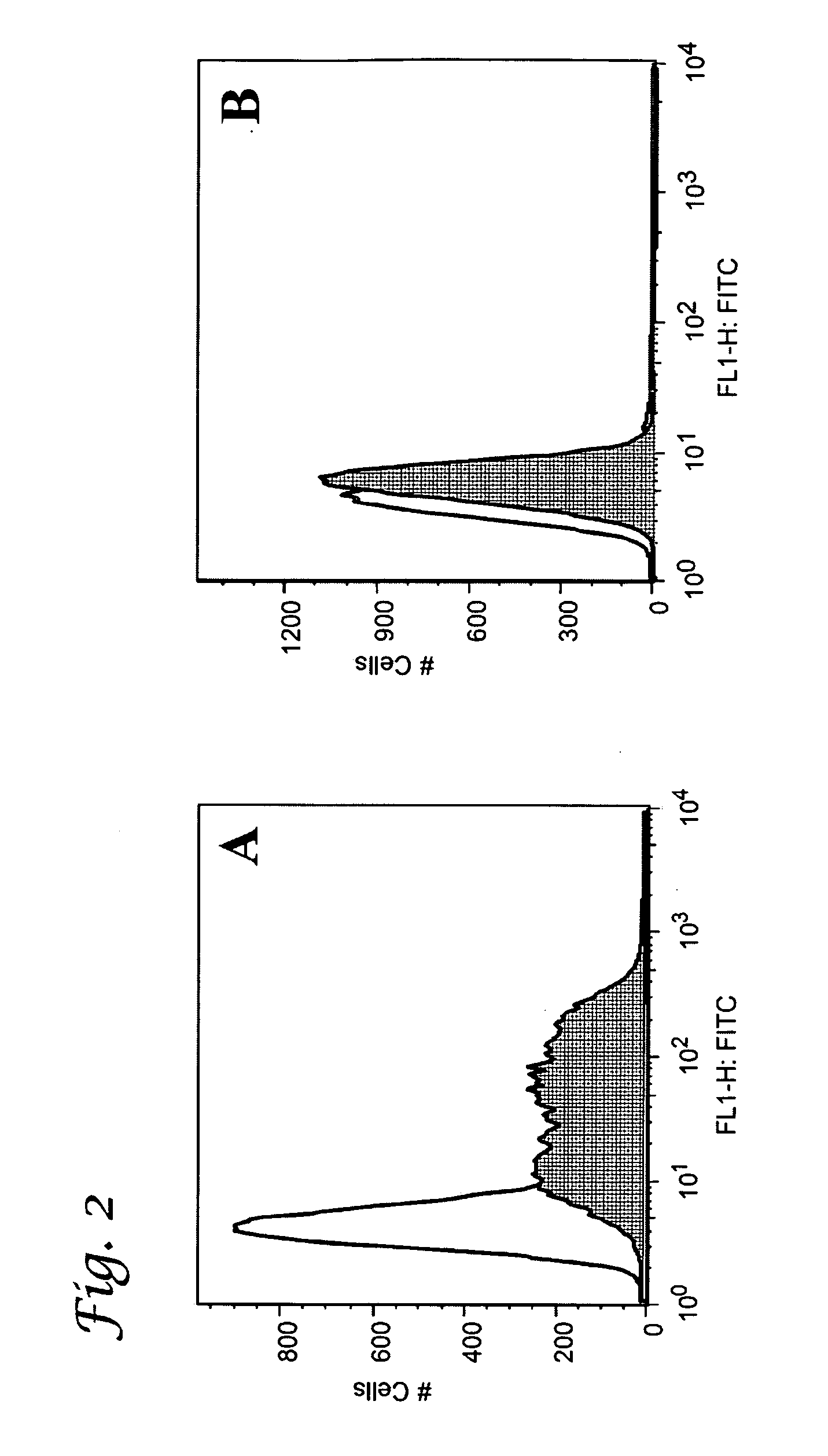
Glycopeptide and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0158]The present invention is illustrated by the following examples. It is to be understood that the particular examples, materials, amounts, and procedures are to be interpreted broadly in accordance with the scope and spirit of the invention as set forth herein.
example i
Towards a Fully Synthetic Carbohydrate-Based Anti-Cancer Vaccine: Synthesis and Immunological Evaluation of a Lipidated Glycopeptide Containing the Tumor-Associated Tn-Antigen
[0159]In this Example, a fully synthetic candidate cancer vaccine, composed of a tumor associated Tn-antigen, a peptide T-epitope and the lipopeptide Pam3Cys was prepared by a combination of polymer-supported and solution phase chemistry. Incorporation of the glycolipopeptide into liposomes gave a formulation that was able to elicit a T-cell dependent antibody response in mice.
[0160]A common feature of oncogenic transformed cells is the over-expression of oligosaccharides, such as Globo-H, LewisY, and Tn antigens (Lloyd, Am. J Clin. Pathol. 1987, 87, 129; Feizi et al., Trends in Biochem. Sci. 1985, 10, 24-29; Springer, J. Mol. Med. 1997, 75, 594-602; Hakomori, Acta Anat. 1998, 161, 79-90). Numerous studies have shown that this abnormal glycosylation can promote metastasis (Sanders et al., Mol. Pathol. 1999, 52,...
example ii
Non-Covalently Linked Diepitope Liposome Preparations
[0180]In a first set of experiments, the tumor-related carbohydrate B-epitope and the universal T-epitope peptide were incorporated separately into preformed liposomes to form a diepitopic construct. Additionally, the lipopeptide Pam3Cys was incorporated into the liposome with the expectation that it would function as a built-in adjuvant, and thus circumvent the necessity of using an additional external adjuvant, such as QS-21.
[0181]The liposomes were prepared from lipid anchors carrying two different thiol-reactive functionalities, maleimide and bromoacetyl, at their surface. The Pam3Cys adjuvant was also incorporated into the preformed liposome and included a maleimide functionality. Conveniently, the maleimide and the bromoacetyl group show a marked difference in their reactivity towards sulfhydryl groups. The maleimide reacts rapidly with a sulfhydryl compound at pH 6.5, whereas the bromoacetyl requires slightly higher pH 8-9 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com