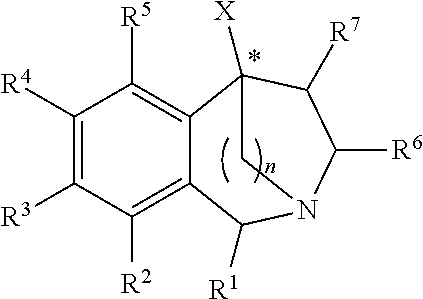
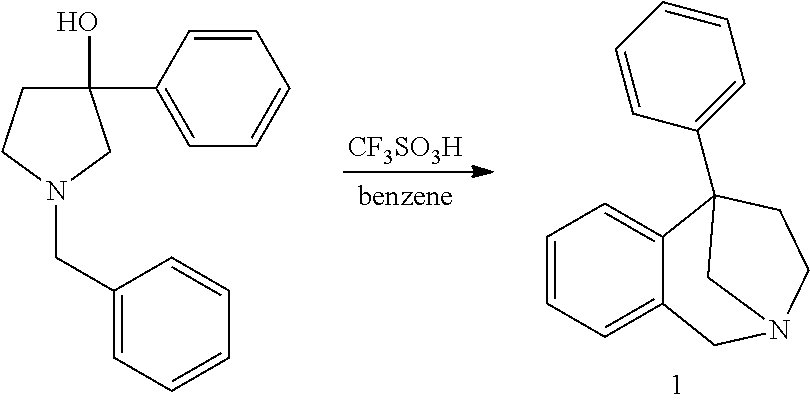
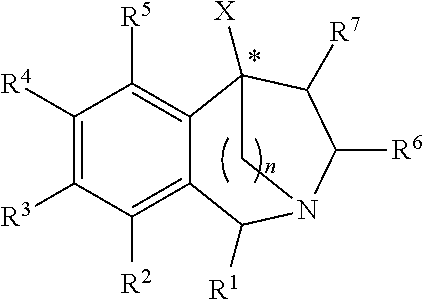
2,5-Methano- and 2,5-Ethano-Tetrahydrobenzazepine Derivatives And Use Thereof To Block Reuptake Of Norepinephrine, Dopamine, and Serotonin
a technology of ethanotetrahydrobenzazepine and ethanotetrahydrobenzazepine, which is applied in the field of compounds, can solve the problems of unresponsive subset of the population, slow onset of action, and common side effects of anticholinergic and sedative drugs,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of rac-5-(4-chlorophenyl)-8-methoxy-2,5-methano-2,3,4,5-tetrahydro-1H-benzo[c]azepine, L-tartrate Salt
[0248]
[0249]Step A: To pyrrolidin-3-ol (3.4 g, 39 mmol) in methanol (150 mL) was added 3-methoxybenzaldehyde (6.4 g, 47 mmol) and iodine (0.40 g, 32 mmol). After stirring for 5 h, the reaction mixture was cooled to 0° C. and sodium borohydride (2.9 g, 78 mmol) was added portionwise. The reaction mixture was warmed to ambient temperature and stirred overnight. Water (10 mL) was added and most of the tetrahydrofuran was removed in vacuo. Additional water (25 mL) was added and the aqueous layer was extracted with methylene chloride (50 mL). The organic layer was dried over sodium sulfate and concentrated in vacuo. The residue was purified by column chromatography (methylene chloride to 90:9:1 methylene chloride / methanol / ammonium hydroxide) to provide 1-(3-methoxybenzyl)pyrrolidin-3-ol (2.0 g, 25%) as a yellow oil: 1H NMR (DMSO-d6, 300 MHz) δ 7.21 (dd, J=8.1, 8.1 Hz, 1H), 6....
example 2
Preparation of rac-5-(4-chlorophenyl)-2,5-methano-2,3,4,5-tetrahydro-1H-benzo[c]azepin-8-ol, L-tartrate Salt
[0254]
[0255]Step A: A mixture of 5-(4-chlorophenyl)-8-methoxy-2,5-methano-2,3,4,5-tetrahydro-1H-benzo[c]azepine (100 mg, 0.33 mmol) from Step D of Example 1 in 48% hydrobromic acid in water (2 mL) and acetic acid (2 mL) was heated to 135° C. After 4 h, the reaction mixture was cooled to ambient temperature, brought to ca. pH 7-8 with 1 N sodium hydroxide and saturated sodium bicarbonate and extracted with ethyl acetate (2×). The combined organics were washed with brine and concentrated in vacuo. The residue was purified by column chromatography (methylene chloride to 90:9:1 methylene chloride / methanol / ammonium hydroxide) to provide 5-(4-chlorophenyl)-2,5-methano-2,3,4,5-tetrahydro-1H-benzo[c]azepin-8-ol (67 mg, 70%) as a white solid.
[0256]Step B: To the benzazepinol (25 mg, 0.087 mmol) from Step A above in methanol (1.5 mL) and acetonitrile (4 mL) was added L-tartaric acid (13...
example 3
Preparation of (+)- and (−)-8-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-5-(4-chlorophenyl)-2,5-methano-2,3,4,5-tetrahydro-1H-benzo[c]azepine, L-tartrate Salts
[0257]
[0258]Step A: To a suspension of 5-(4-chlorophenyl)-2,5-methano-2,3,4,5-tetrahydro-1H-benzo[c]azepin-8-ol (270 mg, 0.94 mmol) from Step A of Example 2 in methylene chloride (10 mL), at −20° C., was added pyridine (0.084 mL, 1.0 mmol) and triflic anhydride (0.018 mL, 1.0 mmol). After 1 h, additional pyridine (0.020 mL, 0.25 mmol) and triflic anhydride (0.031 mL, 0.18 mmol) were added. After 0.5 h at 0° C., additional pyridine (0.020 mL, 0.25 mmol) and triflic anhydride (0.031 mL, 0.18 mmol) were added. After 1.25 h, the reaction mixture was diluted with methylene chloride (20 mL) and saturated sodium bicarbonate (10 mL) was added. The organic layer was separated and washed with brine (10 mL), dried over sodium sulfate and concentrated in vacuo to provide 5-(4-chlorophenyl)-2,5-methano-2,3,4,5-tetrahydro-1H-benzo[c]azepin-8-yl t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com