Method for producing contrast agent for optical imaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Solubility of ICG in Chloroform when Phospholipid was Added
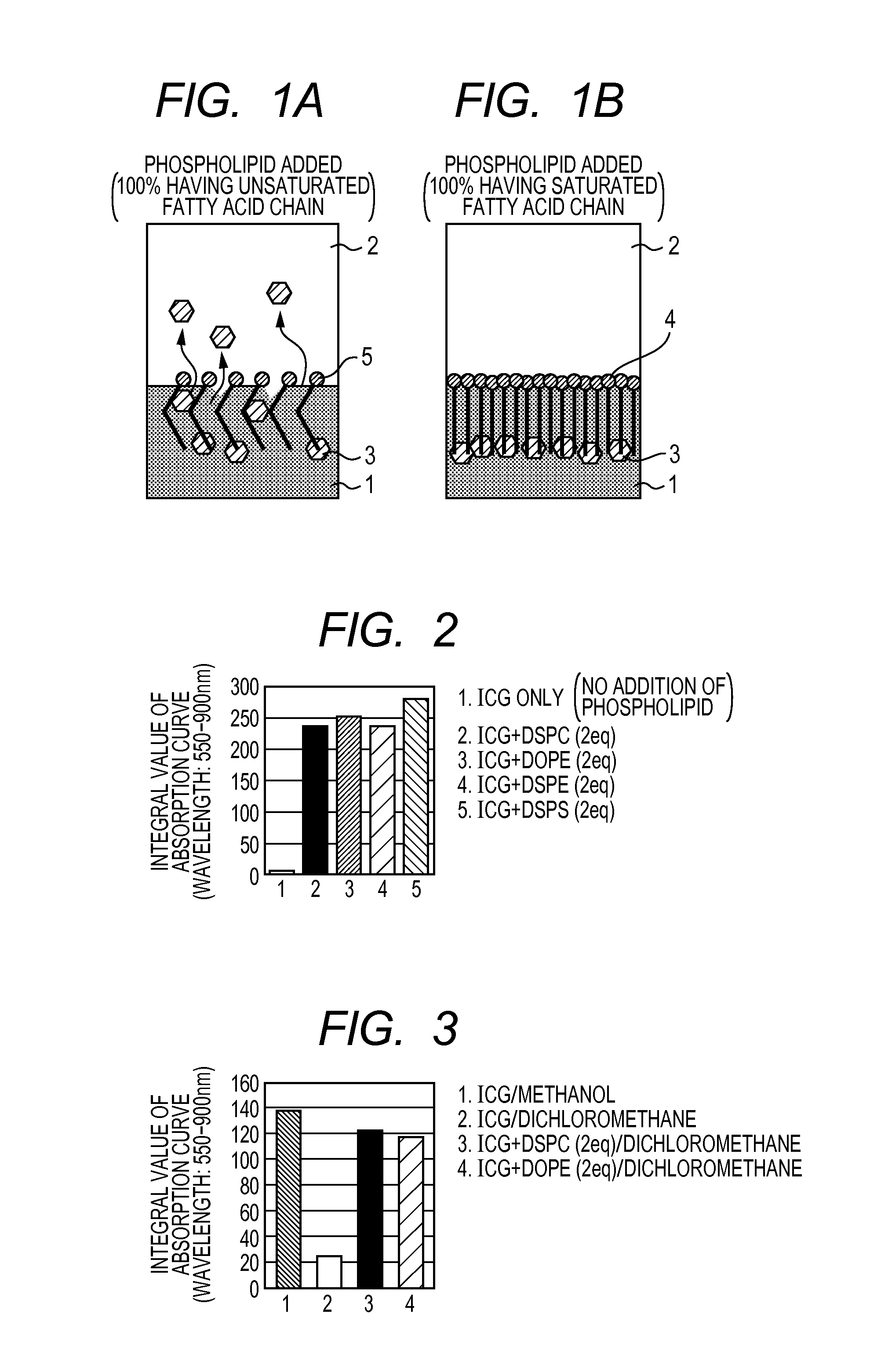
[0077]Double molar equivalents of a phospholipid was added to 5.5 mg of ICG. As the phospholipid, DSPC, DOPE, DSPE or DPPS was used. These ICG-phospholipid mixtures were dissolved in 3 mL of a methanol-chloroform (1:2) mixture. A control was further prepared by dissolving ICG alone in 3 mL of a methanol-chloroform (1:2) mixture. Subsequently, pressure of these solutions was reduced at 40° C. to distill off the solvent. Each of the ICG-phospholipid mixtures evaporated to dryness were dissolved in 2 mL of chloroform and filtered with a filter having a pore size of 0.2 μm. The filtrates were diluted 100-fold with chloroform. Using a quartz cell having a light path of 1 cm, absorbances were measured wavelengths between 550 and 900 nm in intervals of 1 nm. The sums of absorbances wavelengths between 550 and 900 nm are shown in FIG. 2 as the absorption curve integral values.
[0078]Compared with the case where ICG alone was dissolve...
example 2
Solubility of ICG in Dichloromethane when Phospholipid was Added
[0080]2 molar equivalents of a phospholipid was added to 5.5 mg of ICG. As the phospholipid, DSPC or DOPE was used. Each of these ICG-phospholipid mixtures was dissolved in 2 mL of dichloromethane. Controls were brepared by dissolving in 10 G in methanol or dichloromethane. All these 10 G solutions were filtered with a filter having a pore size of 0.2 μm and then diluted 500-fold with dichloromethane. The absorption curve integral values obtained in the same manner as in Example 1 are shown in FIG. 3.
[0081]Compared with the cases where ICG alone was dissolved in dichloromethane, the solubility of ICG in dichloromethane was improved approximately six times when DSPC or DOPE was added, and ICG was dissolved to a similar extent as the ICG dissolved methanol. Furthermore, the absorption curve integral values of the ICG-methanol solution (2.75 mg / mL=3.5 mM) and the absorption curve integral values of the ICG-dichloromethane ...
reference example 1
Leakage of ICG Dissolved in Dichloromethane-Methanol Mixture into Water
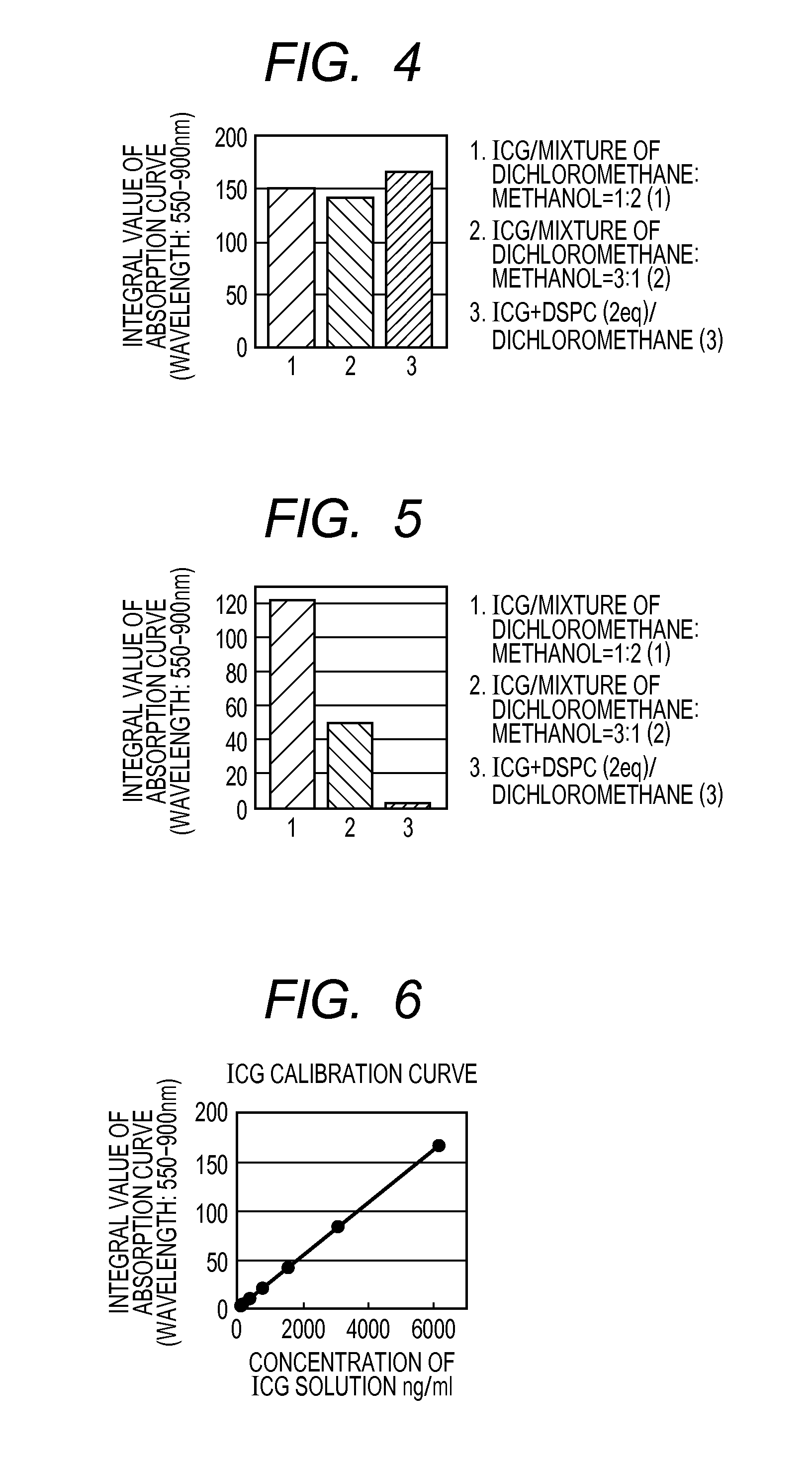
[0082](Preparation of ICG Solution)
[0083]11 mg of ICG was dissolved in 4 rut of a dichloromethane-methanol (1.2) mixture or a dichloromethane-methanol (3:1) mixture, and the mixture was filtered with a filter having a pore size of 0.2 μm. The obtained filtrates were designated as ICG solutions (1) and (2).
[0084]DSPC was added to 11 mg of ICG in a molar ratio of 1:2, and the mixture was dissolved in 3 mL of a methanol-chloroform (1:2) mixture. Subsequently, the solvent of this solution was distilled off under reduced pressure at 40° C. The ICG-DSPC mixture evaporated to dryness was dissolved in 4 mL of dichloromethane and, the mixture was filtered with a filter having a pore size of 0.2 μm. The filtrate was designated as ICG solution (3).
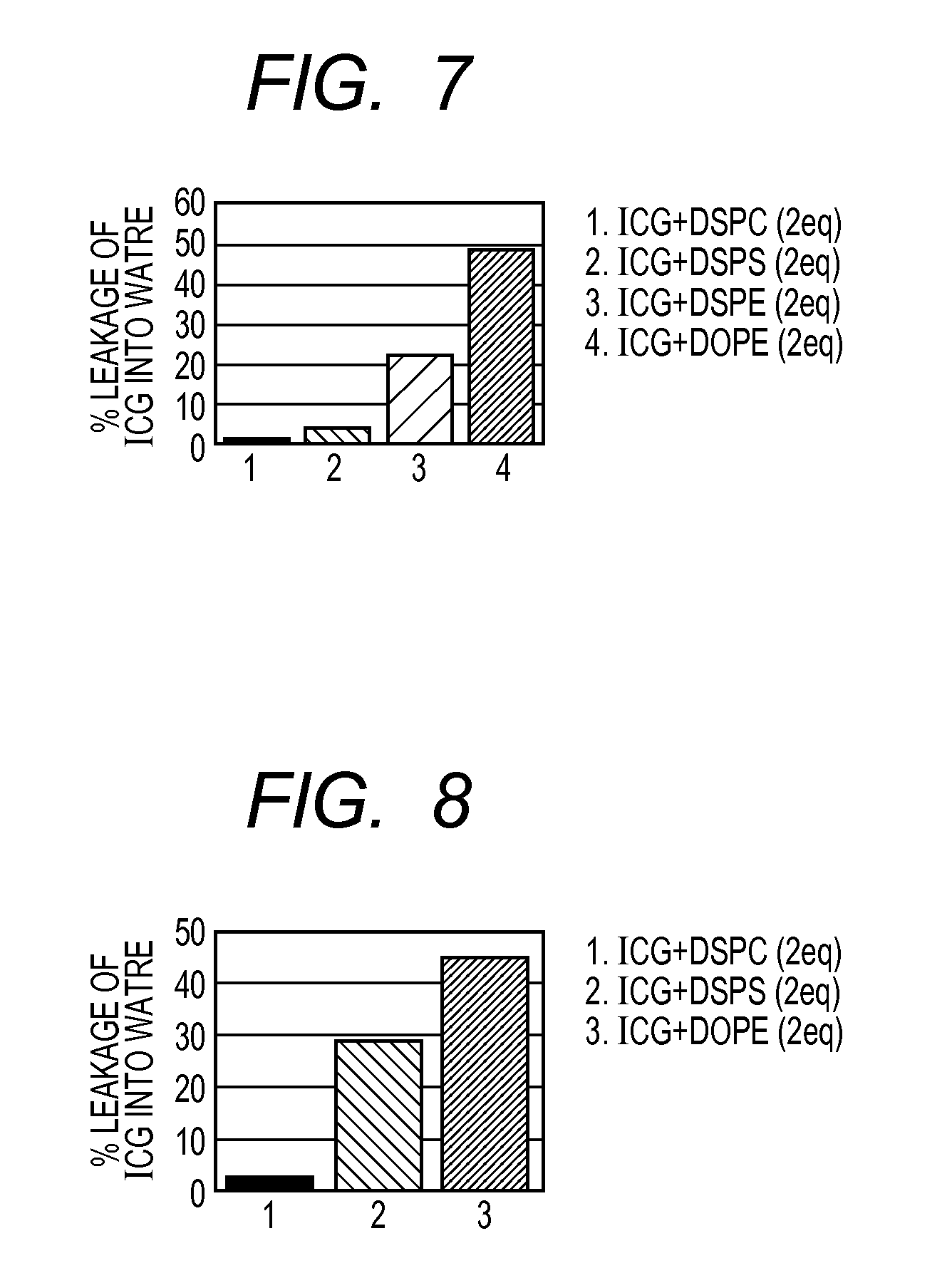
[0085]Each of the ICG solutions (1) to (3) was diluted 500-fold with dichloromethane, and absorption curve integral values were obtained in the same manner as in Example 1. The re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com