Compositions and Methods for Implantation of Processed Adipose Tissue and Processed Adipose Tissue Products
a technology of adipose tissue and adipose tissue products, applied in the field of regenerative medicine, can solve the problems of requiring significant time for new tissue formation, method cost, and procedure limitations, and achieve the effect of reducing the lifetime of implanted processed adipose tissu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a Cellular Biocompatible Biomaterial
[0196]A lipoaspirate was obtained using standard minimally invasive surgical techniques. Tumescent fluid was removed and the lipoaspirate was placed on ice until next step. The lipoaspirate was combined with various surfactants and scaffolds for the preparation of the biocompatible biomaterial.
[0197]1. Hyaluronic acid was emulsified with cellularized lipoaspirate in a 1:1 ratio with the addition of 5% Pluronic surfactant.
[0198]2. A 10% weight per volume PEG-DA was dissolved in a 50:50 ratio of PBS:Lipoaspirate.
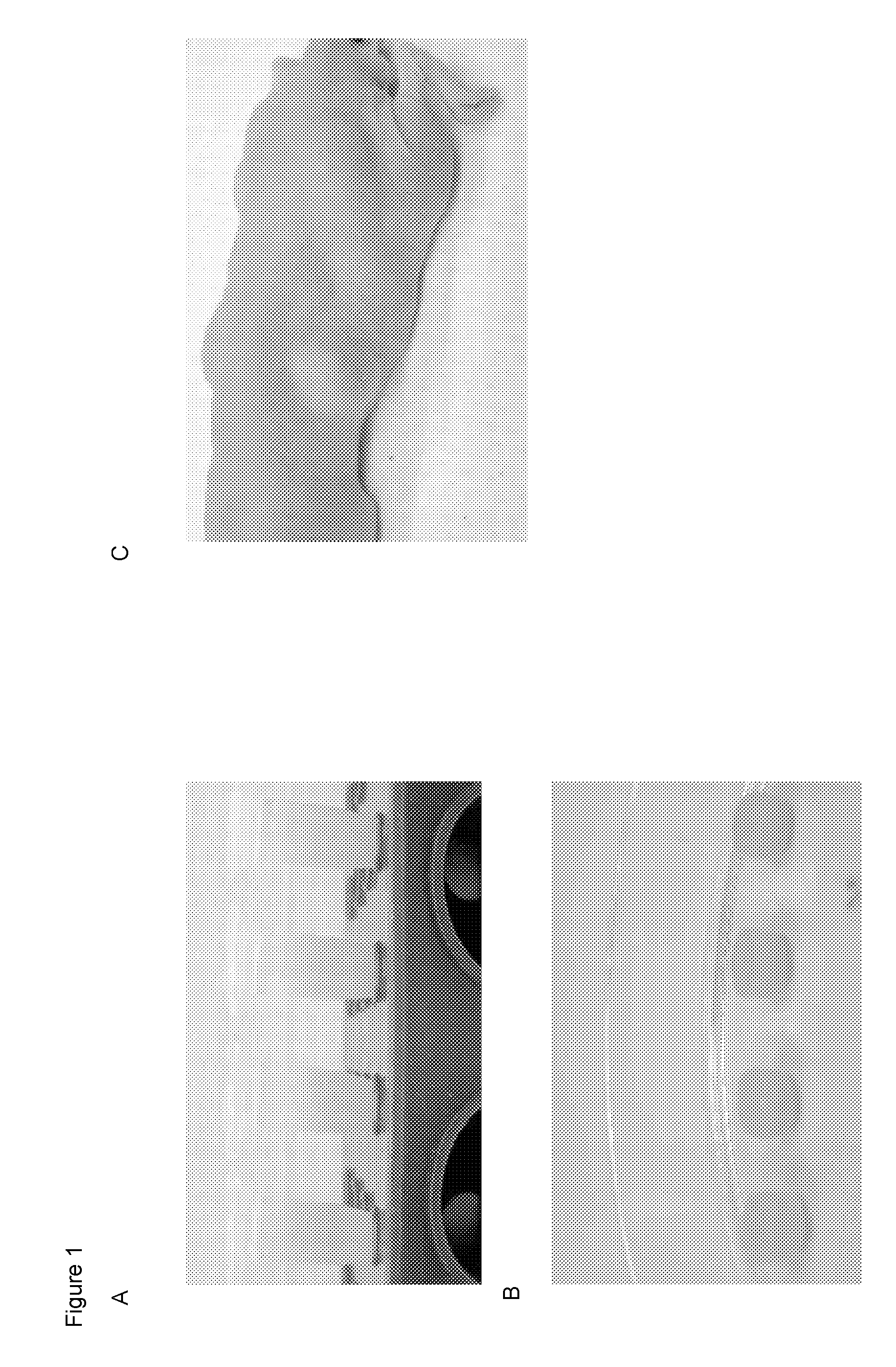
[0199]FIG. 1 shows emulsification of the lipoaspirate and aqueous PEG mixture in the absence or presence of surfactant. In FIG. 1A, the far left tube contains no surfactant. Phase separation between the aqueous and lipid layers of the mixture is evident. Moving to the right, increased concentrations of surfactant were added, and improved emulsification was observed.
[0200]The HA used was a commercially available crosslinked HA ...
example 2
Preparation of Acellular Biomaterial / Processed human Adipose Tissue (PhAT)
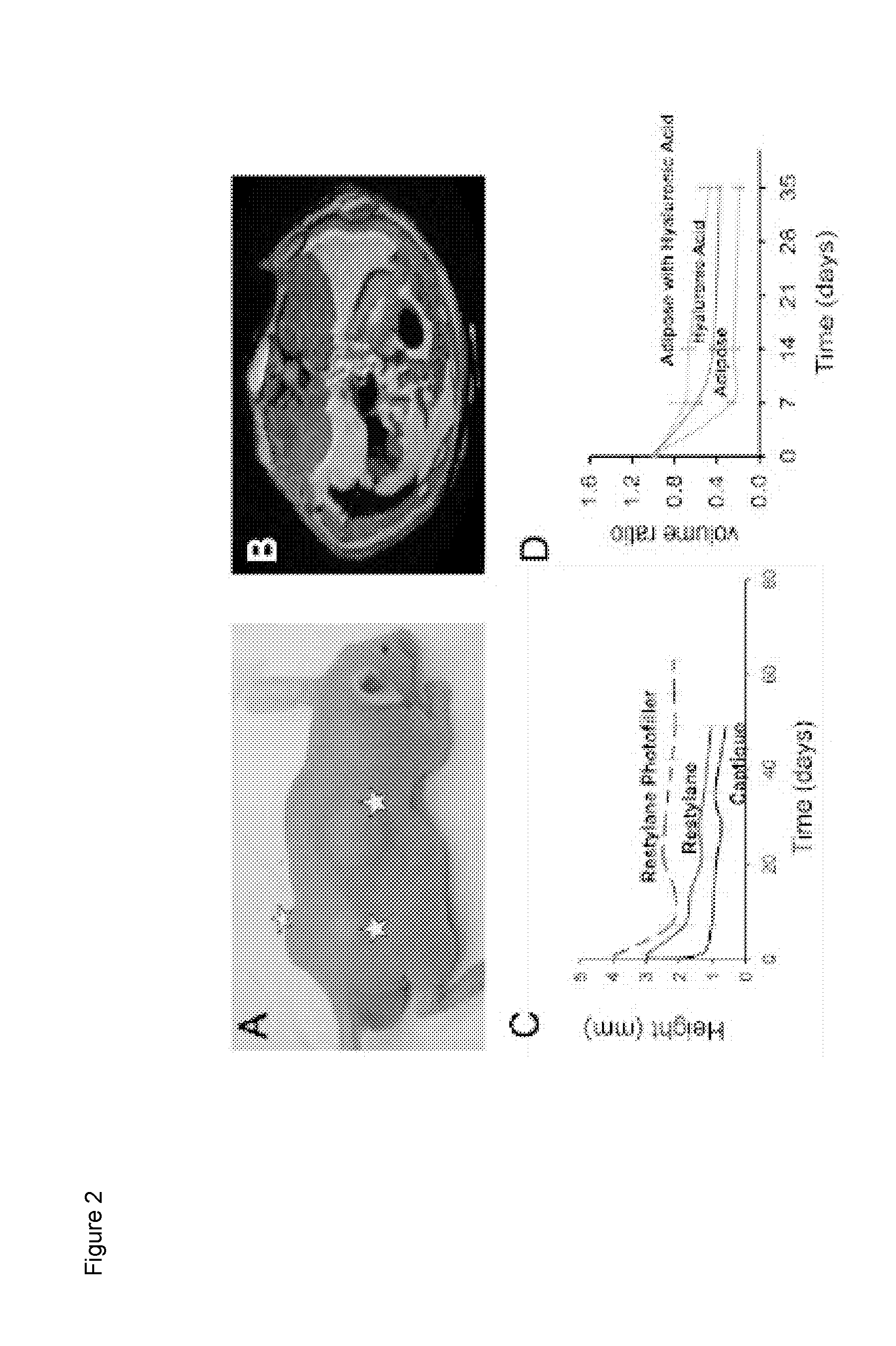
[0206]Tissue acquisition and processing. Tissue was acquired from fresh surgical and cadaveric sources with appropriate consent of the donors. A representative sample of subcutaneous fat is shown in FIGS. 3A and 9A. A representative histological section showing nuclei, lipid vacuoles, and extracellular matrix is provided in FIGS. 3C and 9C.
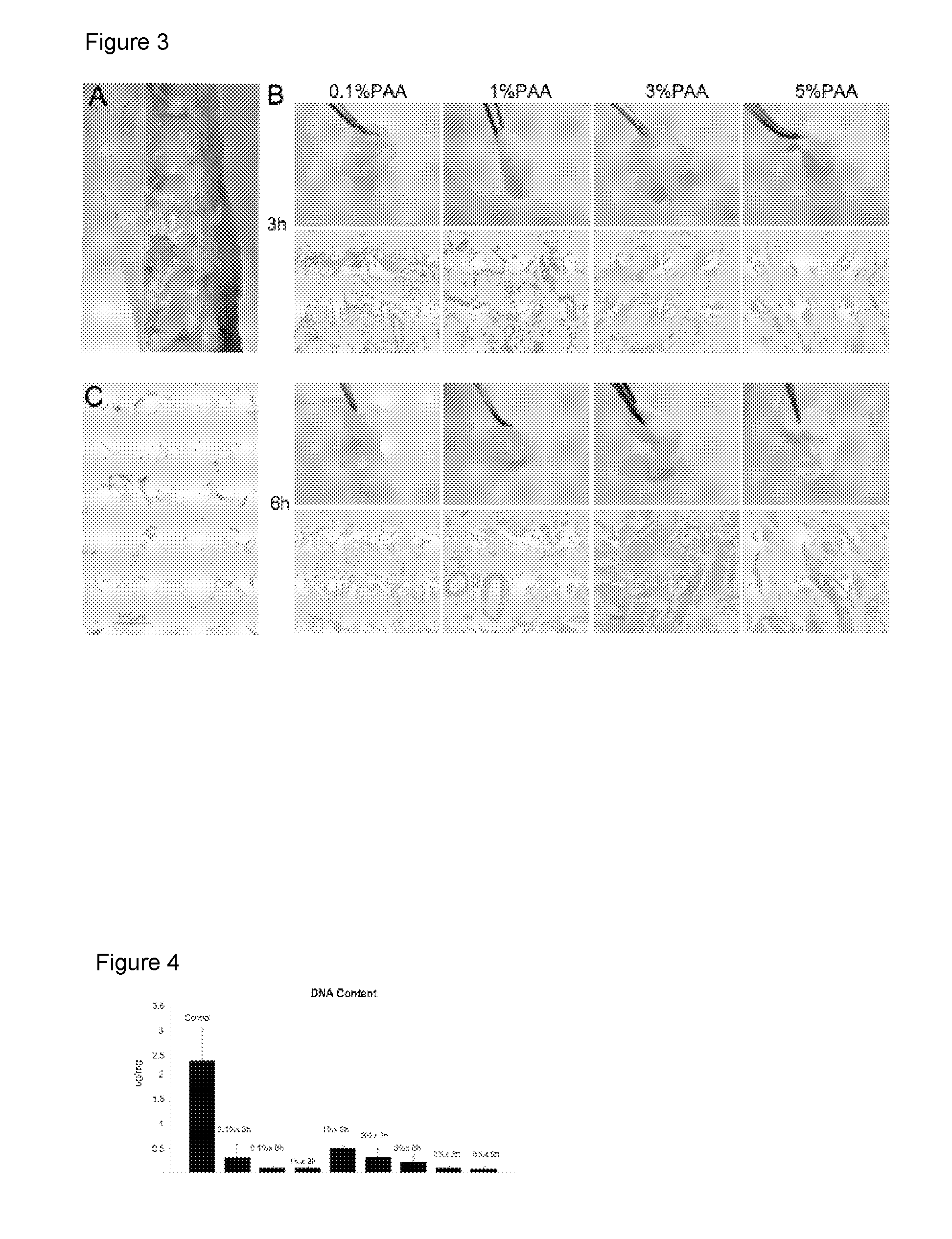
[0207]Subcutaneous fat was isolated from the sample by scraping. The scraped adipose tissue was homogenized in a blender. The homogenate was then placed on a strainer and washed for 5 minutes under deionized water to wash lipid and cellular debris. This was repeated three times. An equal weight of the homogenized and washed tissue was placed in 0.1%, 1%, 3%, or 5% peracetic acid for 3 or 6 hours on a shaker at 37° C. As adipocytes die, oil is released from the cells. To fully infiltrate the adipose tissue and remove the oil, the material was manually manipulated with a morta...
example 3
Analysis of Acellular Biocompatible Biomaterial
[0215]The acellular biocompatible biomaterial (processed human adipose tissue or PhAT) was tested using a number of assays to demonstrate that the material is acellular, lipid-free, includes intact extracellular matrix (ECM), and the appropriate dynamic stiffness. Exemplary assays used are provided. Other methods to determine if the material has the desired characteristics are known in the art.
[0216]Cell Free. Hematoxylin and eosin (H&E) staining was performed on paraffin-embedded sections of PAT prepared by at least one of the methods of the previous example to determine the presence of nuclei (cells) (compare FIGS. 7C and 9C to FIGS. 7B and 9D). No cellular material or nuclei were observed after processing with PAA and DNAse (FIG. 7B) or with PAA, TX-100, and DNAse (FIG. 9D) per the methods of the invention.
[0217]In addition, MHC class I immunostaining can be performed to evaluate the presence of antigens.
[0218]DNA content: To demonst...
PUM
| Property | Measurement | Unit |
|---|---|---|
| elastic modulus | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com