Micro-RNA Biomarkers and Methods of Using Same
a microrna biomarker and biomarker technology, applied in the field of microrna biomarkers and methods of using same, can solve the problem of low healing rate of non-small cell lung carcinoma (nsclc)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Studies, Materials & Methods
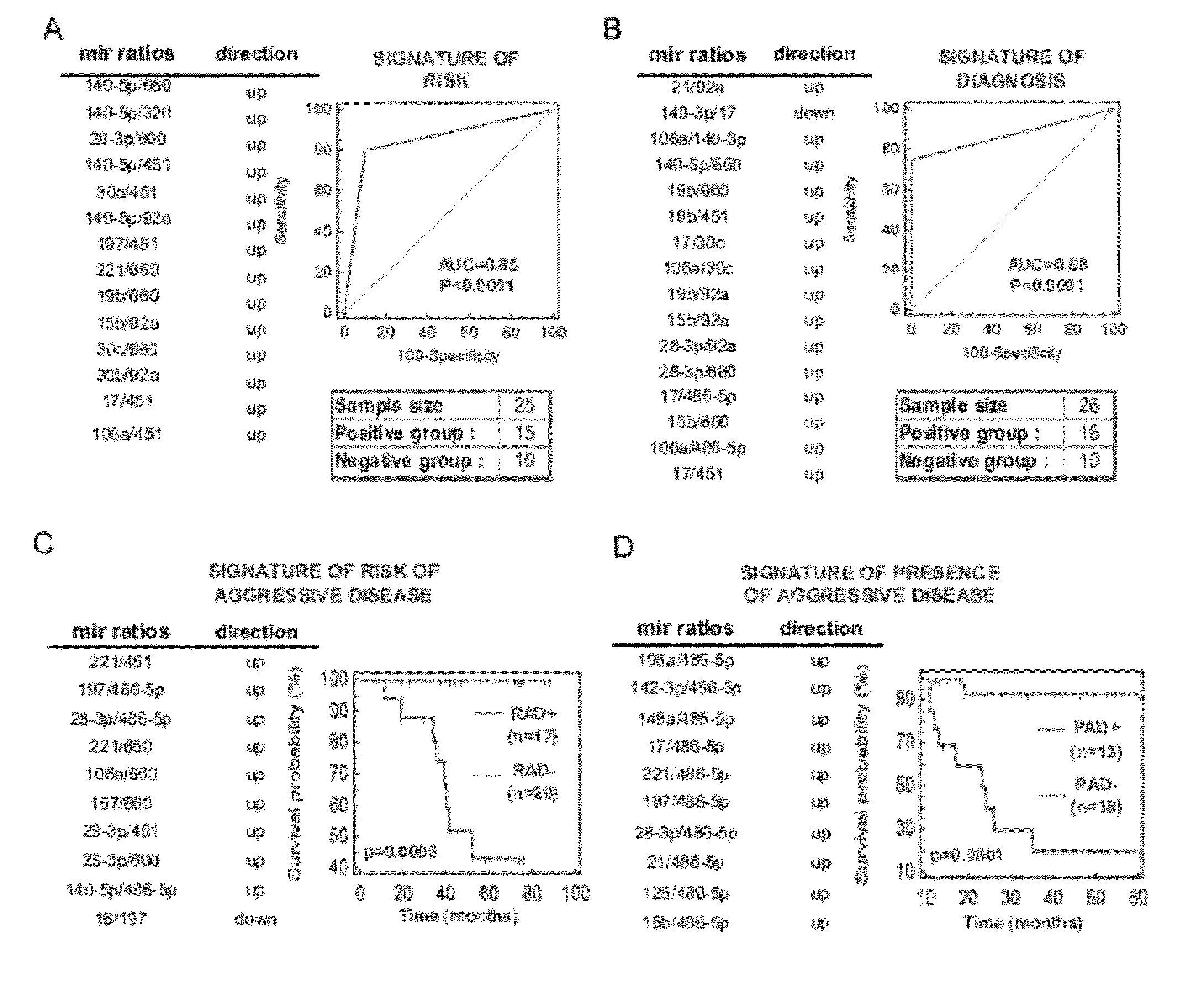
[0226]The present invention investigated the expression profile of miRNA in the plasma of individuals enrolled in screening protocols using spiral CT. This investigation was done with the aim of verifying the capability of miRNAs as a new class of biomolecular markers for: prediction of the risk of developing a tumour, in particular a pulmonary tumour, and diagnosis of the tumour, in particular pulmonary tumour, and thus as a prognostic aid for discriminating patients with indolent or aggressive pulmonary lesions.
[0227]Plasma samples taken from smoker individuals were used, where the individuals were over 50 years old, in a time parameter of between one and two years before detection with CT spiral of the presence of a pulmonary tumour in the same individuals. Also used were samples of plasma collected at the moment of the appearance of the disease (detected using spiral CT). The plasma samples were obtained from patients who had developed a pulmonary tum...
example 2
miRNA Treatment
[0313]The instant example demonstrates modifying the level of two microRNAs of our plasma signatures in a lung cancer cell line (A549). Mir-486 and mir-660 were down-modulated in plasma samples of patients with lung cancer and in particular in those who have developed the aggressive form of the disease. In FIG. 9 microRNA levels were measured by qReal-Time PCR in 20 paired tumor and normal lung tissue of the same patients enrolled in the CT-screening trial used as validation set. Row Ct data were normalized on the housekeeping miRNA RNU6B (DCt). The final expression values were obtained with the formula: 2̂(−DCt of the tumor tissue) / 2̂(−DCt of the normal lung). Values >1→upregulated in tumor tissue. Values<1→downregulated in tumor tissue. The results in FIG. 9 show that these two miRNA were downregulated in the tumor tissue compared with the normal lung tissue.
[0314]In FIG. 10 mirVana™ miRNA Mimic (Applied biosystem) were used to transfect lung cancer cell line expres...
example 3
Lung Cancer Detection and Survival
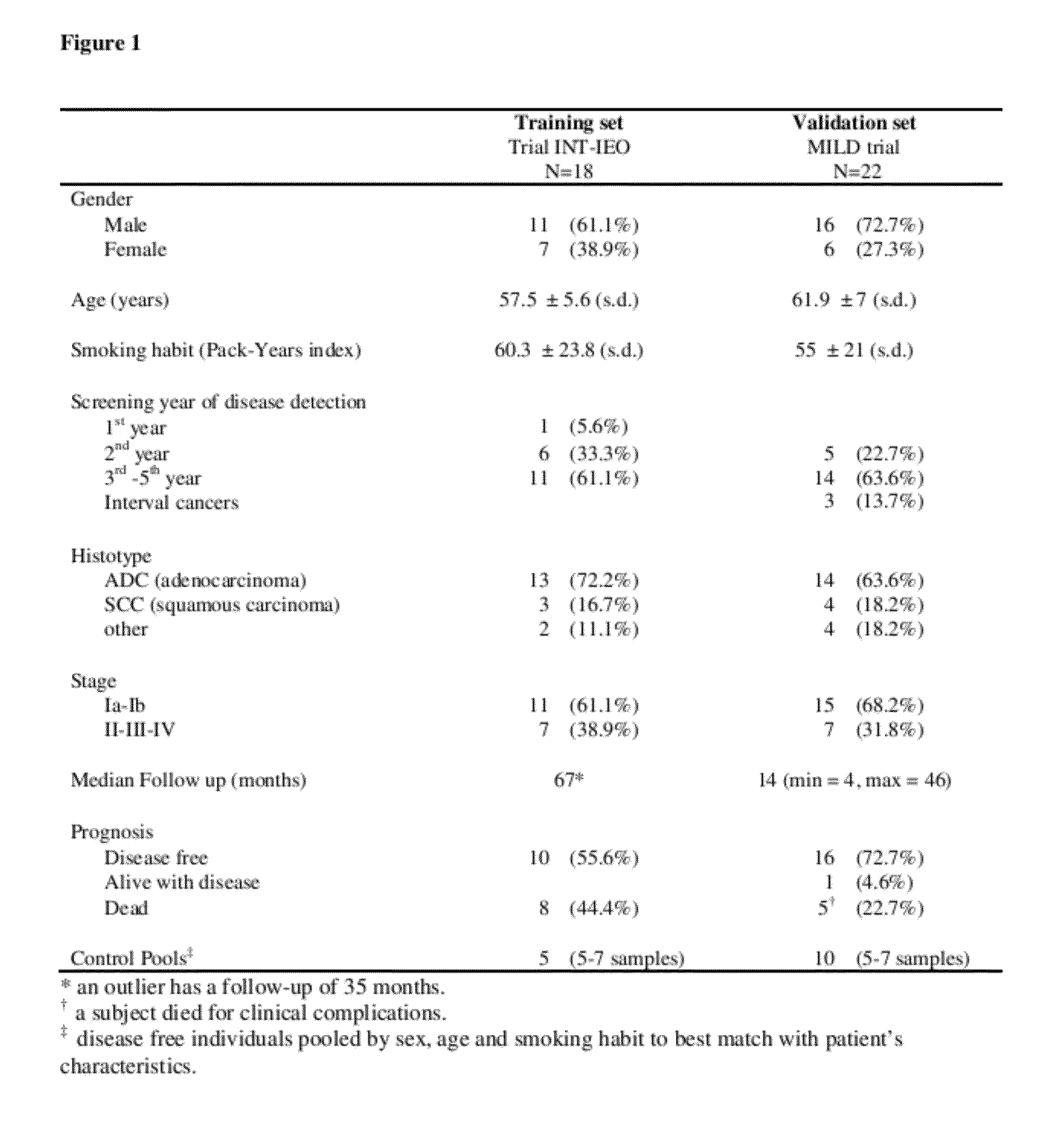
[0317]INT-IEO cohort (training set). Lung cancer was diagnosed in 38 subjects, 22 in the first 2 y and 16 from the 3rd to 5th y of screening, including one interval cancer at 4th y. The frequency of stage I was 63% (77% in first 2 y vs. 44% in the last 3 y), and adenocarcinoma was 71% (95% in first 2 y vs. 63% in the last 3 y; Table XI).
TABLE XICT YearCharacteristic1-23-5TotalLung Cancer221638Resected21 (95)12 (75)33* (87) Stage I17 (77) 7 (44)24 (63)Stage II-IV 5 (23) 9 (56)14 (37)Adeno17 (95)10 (63)27 (71)*28 tumor tissue and 24 normal lung samples were available for miRNA expression analysis. The number in parenthesis is the percent of all detected lung cancers.
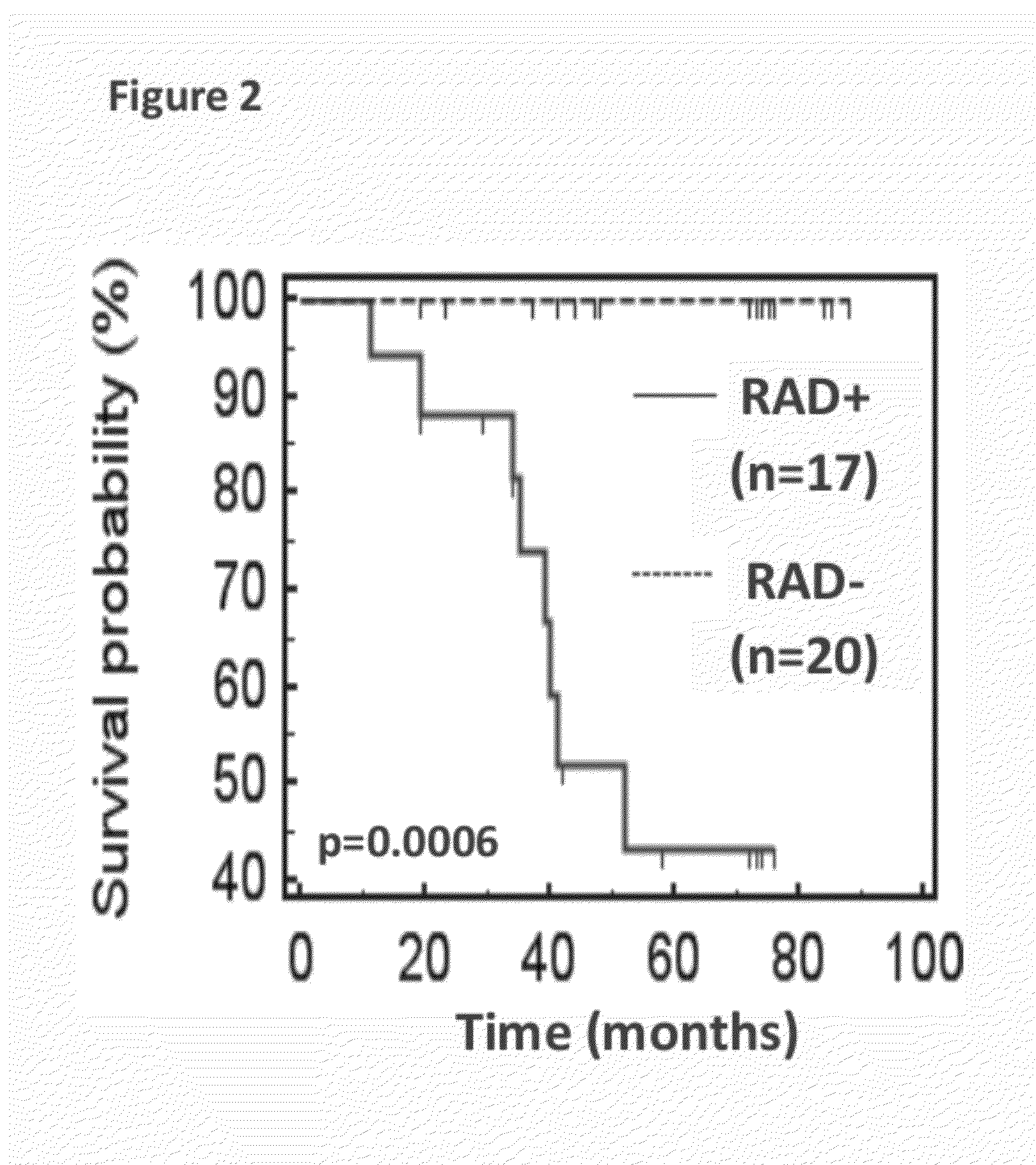
[0318]Median follow-up time for the 38 lung cancer cases was 75 mo, with 60% 5-y overall survival (95% C.I.: 43-74%). Five-y overall survival was 92% for stage 1 and 7% for stages II-IV (P2 test, P=0.034). In the subset of CT year 1-2 / stage I, 5-y survival was 94% (95% C.I.: 65.0-99.1). In...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com