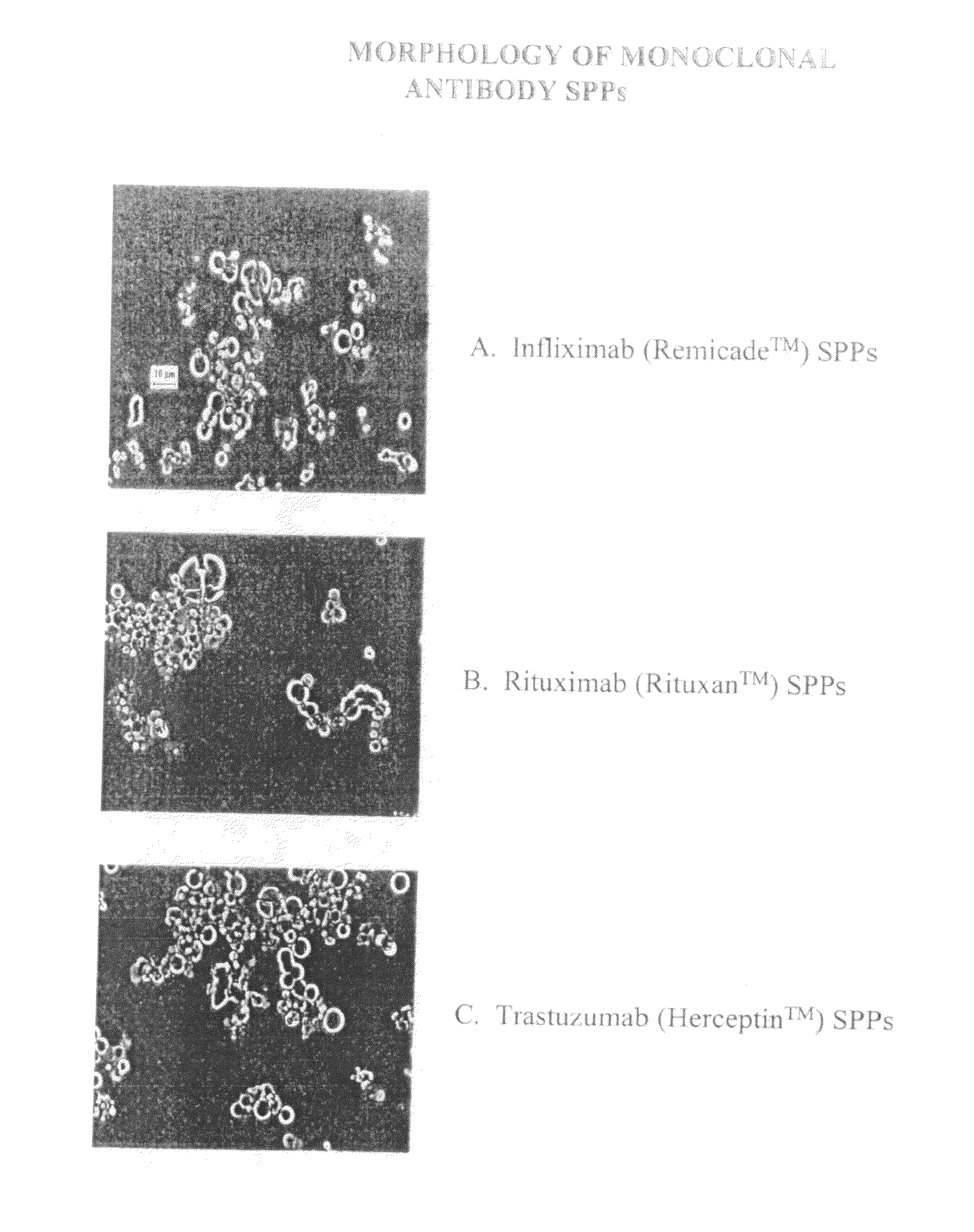
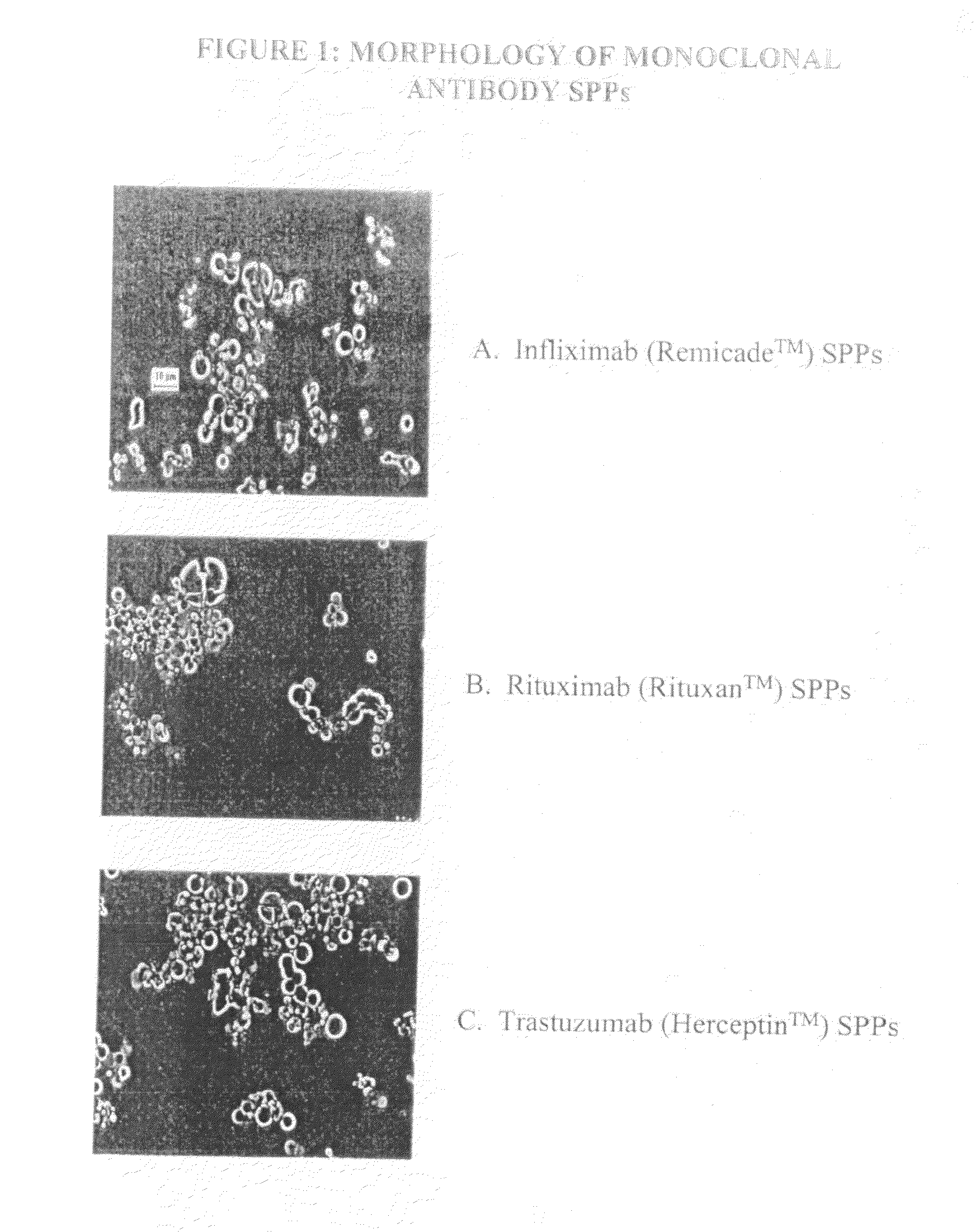
Spherical Protein Particles and Methods of Making and Using Them
a protein particle and spherical technology, applied in the field of spherical protein particles, can solve the problems of poor bioavailability, high cost and time consumption of medical care for patients who require parenteral administration of protein drugs, and difficult patient compliance, and achieve simple, efficient and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Spherical Protein Particles of Infliximab
[0237]Infliximab is a chimeric murine / human monoclonal antibody commercially available as Remicade™ (Centocor, Leiden, the Netherlands). This monoclonal antibody has been widely used to treat rheumatoid arthritis and Crohn's disease. Infliximab is a chimeric IgG1 kappa immunoglobulin that binds to the TNFα antigen. It is composed of murine light- and heavy-chain variable region sequences and a human constant region sequence. The Infliximab antibody has an approximate molecular weight (MWt) of 149 kD.
Infliximab SPP Preparation
[0238]Materials:
[0239]Infliximab antibody (each vial contains 100 mg Infliximab, 500 mg sucrose, 0.5 mg polysorbate 80, 2.2 mg monobasic sodium phosphate and 6.1 mg dibasic sodium phosphate) reconstituted in 10 ml water, pH approximately 7.2 (concentration equal to 10 mg / ml).
[0240]Procedure:
[0241]Infliximab SPPs were formed using a Slide-A-Lyzer (Pierce Chemicals, Catalog #69570), which was used as follows:...
example 2
Preparation of Spherical Protein Particles of Rituximab
[0253]Rituximab is a chimeric murine / human monoclonal antibody commercially available as Rituxan™ (Genentech, Inc., South San Francisco, Calif.). This monoclonal antibody has been widely used to treat non-Hodgkins lymphoma. Rituximab is a chimeric IgG1 kappa immunoglobulin that binds to the CD20 antigen on the surface of normal and malignant B-lymphocytes. It is composed of murine light- and heavy-chain variable region sequences and a human constant region sequence. The Rituximab antibody has an approximate molecular weight (MWt) of 145 kD.
Rituximab SPP Preparation
[0254]Materials:
[0255]Rituximab antibody (stored until use at 4° C., at 10 mg / ml in 9.0 mg / ml sodium chloride, 7.35 mg / ml sodium citrate anhydrate, 0.7 mg / ml Polysorbate 80 and sterile water, pH 6.5)
[0256]Procedure:
[0257]Rituximab SPPs were formed using a 10,000 MW cut-off Slide-A-Lyzer, according to the method described above for Infliximab (Example 1).
[0258]100 μl of...
example 3
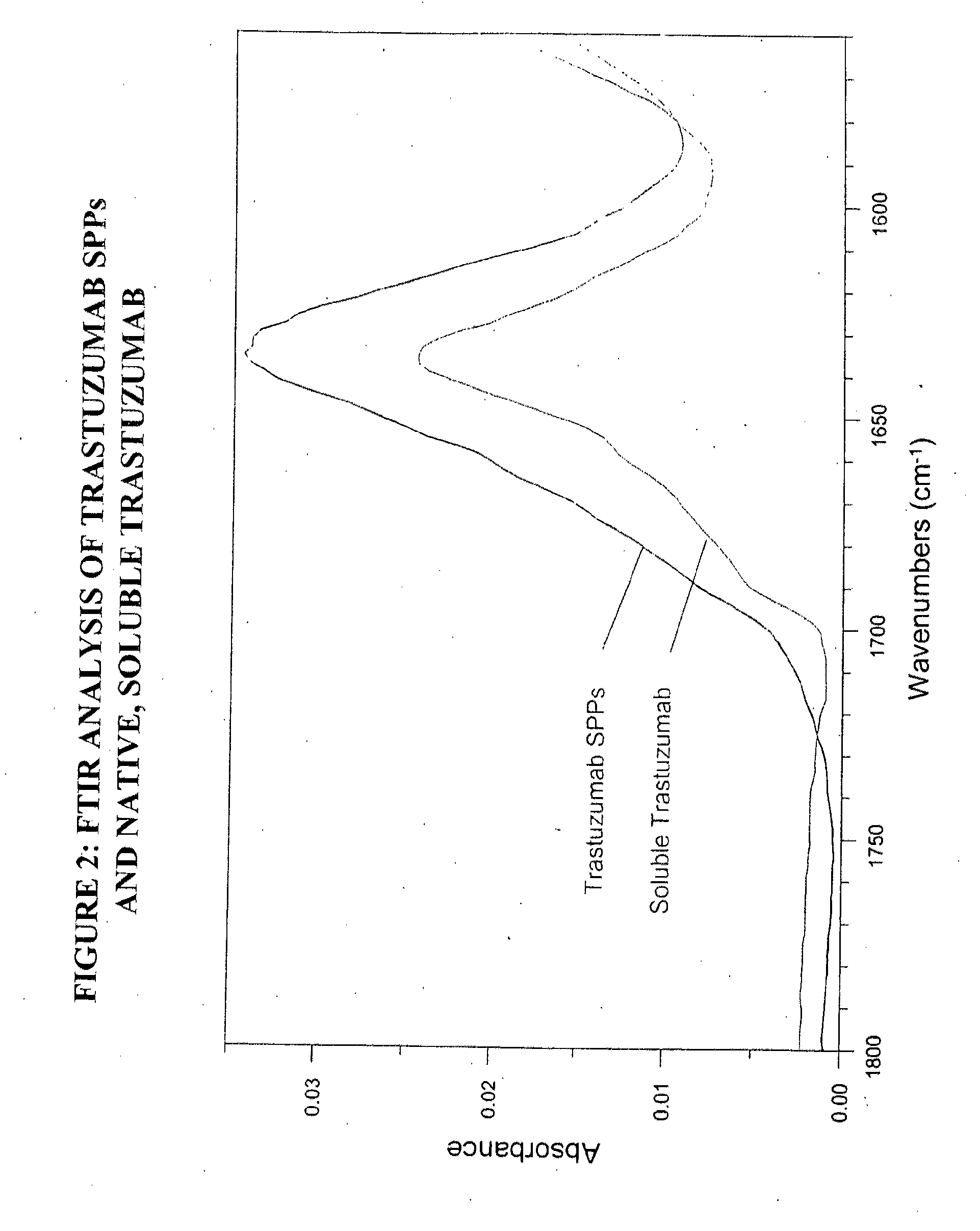
Preparation of Spherical Protein Particles of Trastuzumab
[0261]Trastuzumab is a monoclonal antibody commercially available as Herceptin™ (Genentech, Inc., South San Francisco, Calif.).
Trastuzumab SPP Preparation
[0262]Materials:
[0263]Trastuzumab antibody (available as a lyophilized powder containing 22 mg Trastuzumab, 1 mg L-histidine HCl, 0.64 mg L-Histidine, 40 mg trehalose dihydrate, 0.18 mg polysorbate 20), reconstituted in 1 ml water (22 mg / ml), pH 6.
[0264]Procedure:
[0265]Trastuzumab SPPs were formed using a 10,000 MW cut-off Slide-A-Lyzer, according to the method described above for Example 1.
[0266]100 μl of a Trastuzumab solution (at 22 mg / ml Trastuzumab) was dialyzed against 3.9 ml of a solution consisting of 2.1M ammonium sulfate, 0.1M sodium acetate pH 5.8, 1% propylene glycol. A 10,000 MW cut-off dialysis membrane was used. The mixture was dialyzed at room temperature for 28 hours. Then the protein solution was washed twice in 800 μl of a solution consisting of 2.42 M ammo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com