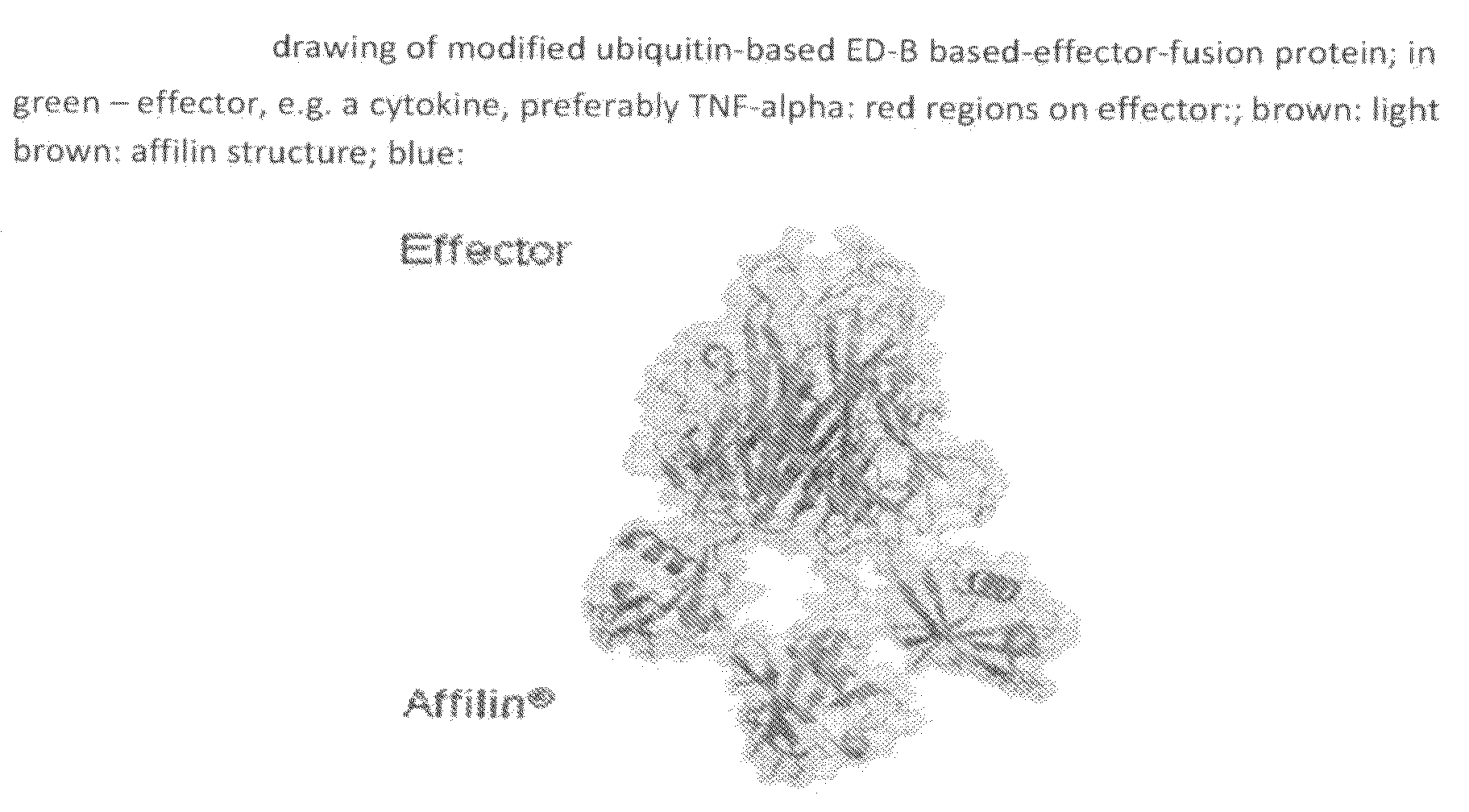
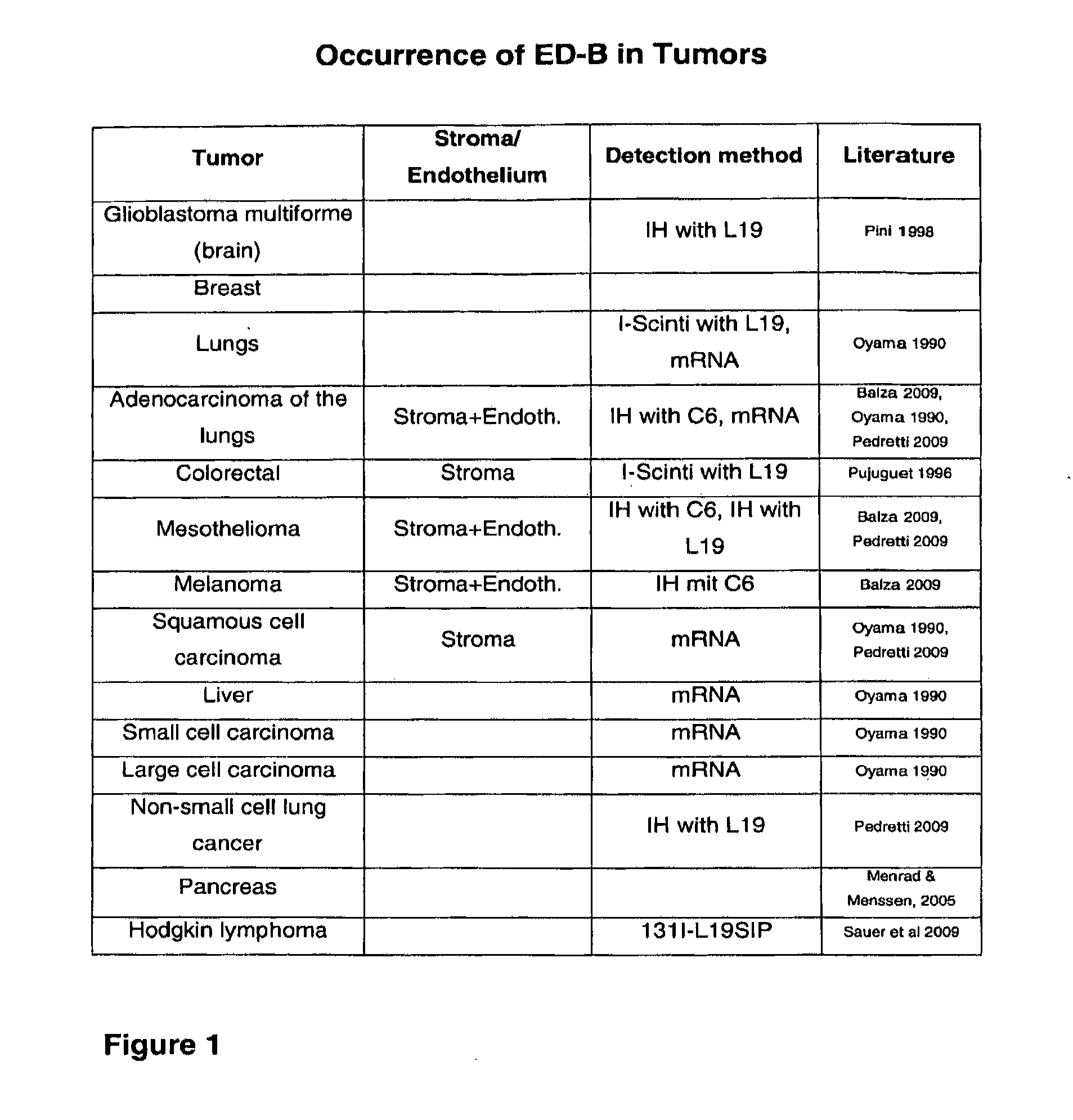
Modified ubiquitin proteins having a specific binding activity for the extradomain b of fibronectin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of ED-B Binding Proteins Based on Modified Ubiquitin Proteins
Library Construction for Monomeric Binding Proteins and Cloning
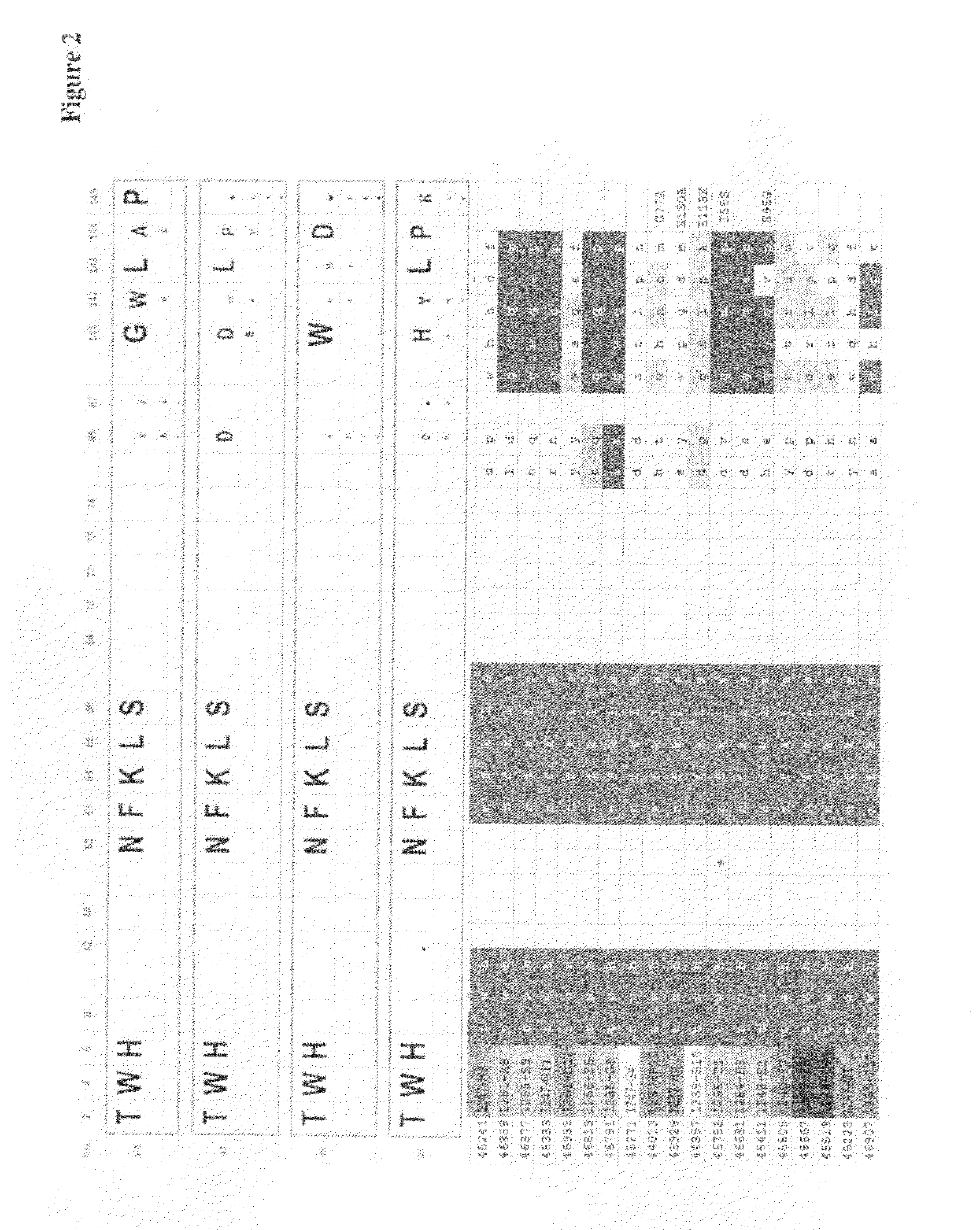
[0240]Unless otherwise indicated, established recombinant genetic methods were used, for example as described in Sambrook et al. A random library of human ubiquitin monomers with high complexity was prepared by concerted mutagenesis of in total up to 10 selected amino acid positions. The modified amino acids, which were substituted by NNK triplets, comprised at least 3 amino acids selected from positions 2, 4, 6, 8, 62, 63, 64, 65, 66, 68 within the ubiquitin monomer.
Library Construction for Hetero-Dimeric Binding Proteins and Cloning
[0241]Unless otherwise indicated, established recombinant genetic methods were used, for example as described in Sambrook et al. A random library of human ubiquitin hetero-dimers with high complexity was prepared by concerted mutagenesis of in total 15 selected amino acid positions. The modified amino acids, which we...
example 2
Production of Fusion Proteins from ED-B-Binding Modified Ubiquitin Variants and TNFalpha (for Example, Human TNFα, Referred to as TNFα)
[0251]The variants can be expressed as fusion proteins between the modified ubiquitins, for example heterodimeric variant1041-D11, and mammalian, for example mouse or human, TNFα in E. coli. Protein analysis of the fusion protein includes: protein expression and purity, no aggregation potential, TNFα activity in cell culture, affinity for target protein ED-B, Selectivity, specific binding in cell culture can be anaylzed. A prerequisite for an animal experiment to induce tumor shrinkage in F9 tumor bearing mice is a fusion with mouse TNFα
Step 1: Production of a Vector for Cloning of Fusion Proteins (pETSUMO— TNFα)
[0252]pETSUMOadapt is a modified vector pETSUMO (Invitrogen), which was modified by insertion of an additional multiple cloning site (MCS). Starting from TNFα cloned in pETSUMOadapt, restriction sites for the insertion of modified ubiquitin v...
example 3
Expression and Purification of Ubiquitin-Based-TNFα Fusion Proteins
[0275]DNA sequence analysis showed the correct sequences of the SUMO-TNFα fusion proteins. For expression of the variants the clones were cultivated in a shaker flask by diluting a preculture 1:100 with LB / Kanamycin and agitating the culture at 200 rpm and 37° C. up to an optical density at 600 nm (OD600) of 0.5. Expression was induced by adding IPTG (final concentration 1 mM). Culturing was continued for 4 hours at 30° C. and 200 rpm. The bacteria cells were harvested by centrifugation at 4° C., 6000×g for 20 min. The cell pellet was suspended in 30 ml of NPI-20 buffer including benzonase and lysozyme. Cells were disrupted by ultrasonication (3×20 sec) on ice. The supernatant containing the soluble proteins was obtained after centrifugation of the suspension at 4° C. and 40000×g for 30 min. Both proteins were purified by affinity chromatography at room temperature. One column of Ni-Agarose (5 ml, GE Healthcare) was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com