Use of bacterial polysaccharides for biofilm inhibition
a technology of bacterial polysaccharides and biofilms, applied in the direction of antibacterial agents, catheter, drug compositions, etc., can solve the problems of biofilm development in industrial devices such as water systems or agri-food plants, raise safety problems, bacteria in biofilms are far more resistant to antibiotics, and are less accessible to the immune system, so as to inhibit biofilm formation and prevent biofilm formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Methods
[0048]Bacterial Strains, Growth Conditions and Microscopy Analysis
[0049]Bacterial strains are listed in Table 1 below. Gram-negative bacteria were grown at 37° C. in M63B1 minimal medium with 0.4% glucose (M63B1 glu) or in LB rich medium. Gram-positive bacteria were grown in TSB with 0.25% glucose (TSBglu) at 37° C. The effect of CFT073 supernatant on bacterial growth and viability rate was evaluated using growth curve determination, colony forming unit count on LB plate and BacLight Live / Dead viability stain (Molecular Probes). Ferritin-staining and Scanning Electronic Microscopy was performed as described in (Bahrani-Mougeot et al., 2002). Epifluorescence and transmitted light microscopy were acquired using a Nikon E400 microscope. Autoaggregation assays were performed as described in (Beloin et al., 2006).
TABLE 1Strains used in this studyStrainsRelevant characteristicsReferencesE. coli strainsCFT073UPEC group II capsule (K2)(Mobley et al., 1990)MG1655F′MG1655 F′tet-ΔtraD p...
example 2
Anti-biofilm Activity of CFT073 Supernatant
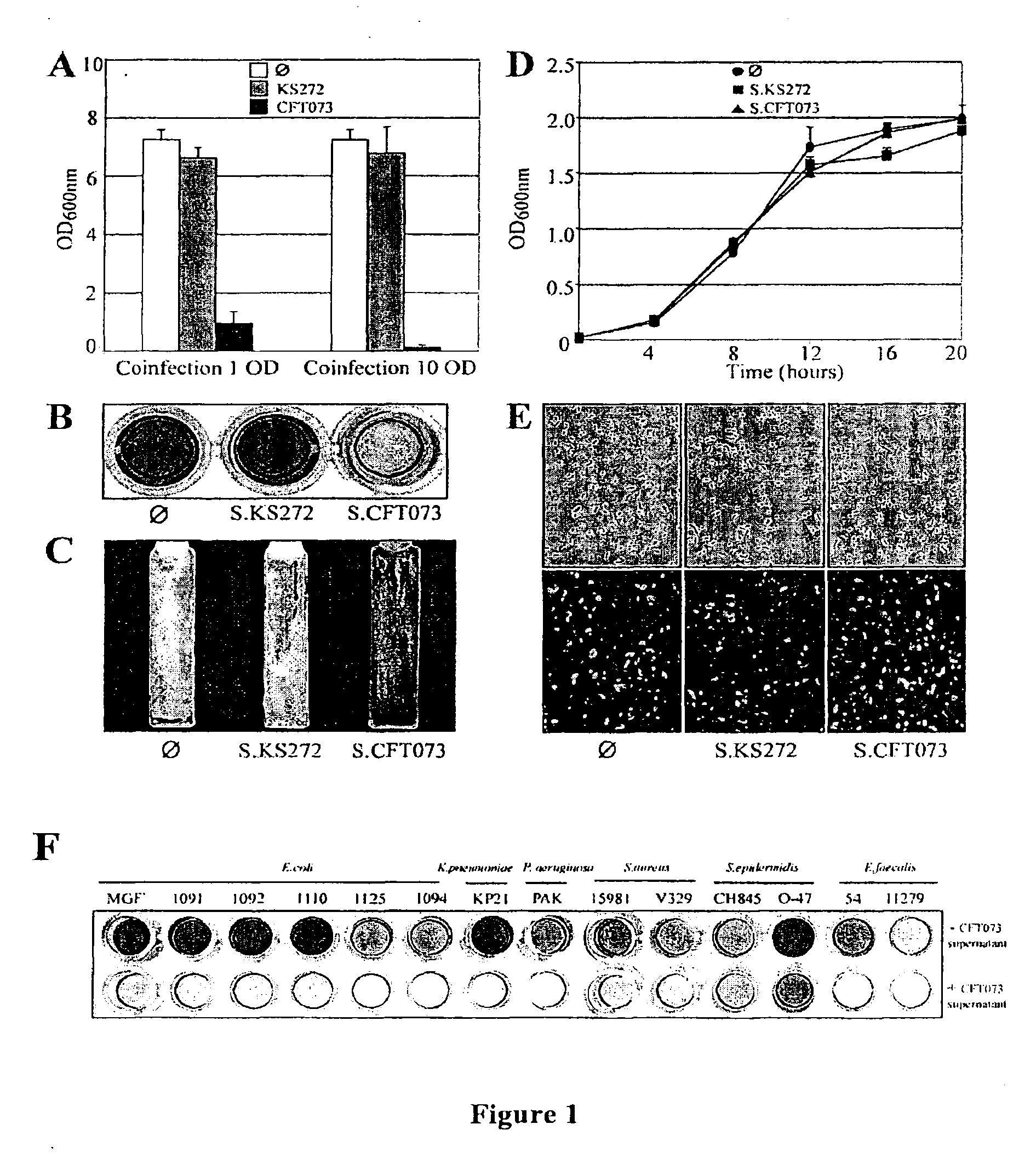
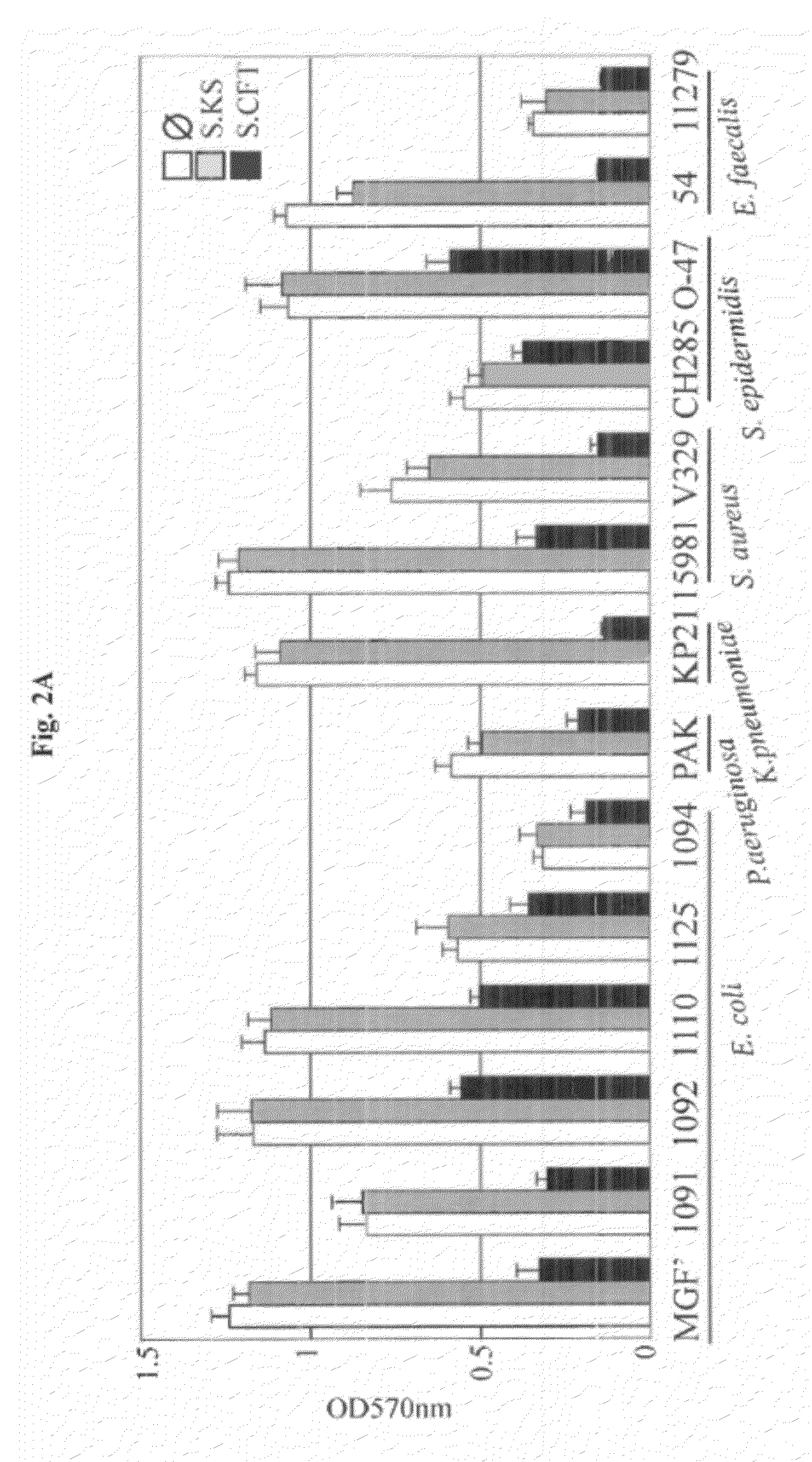
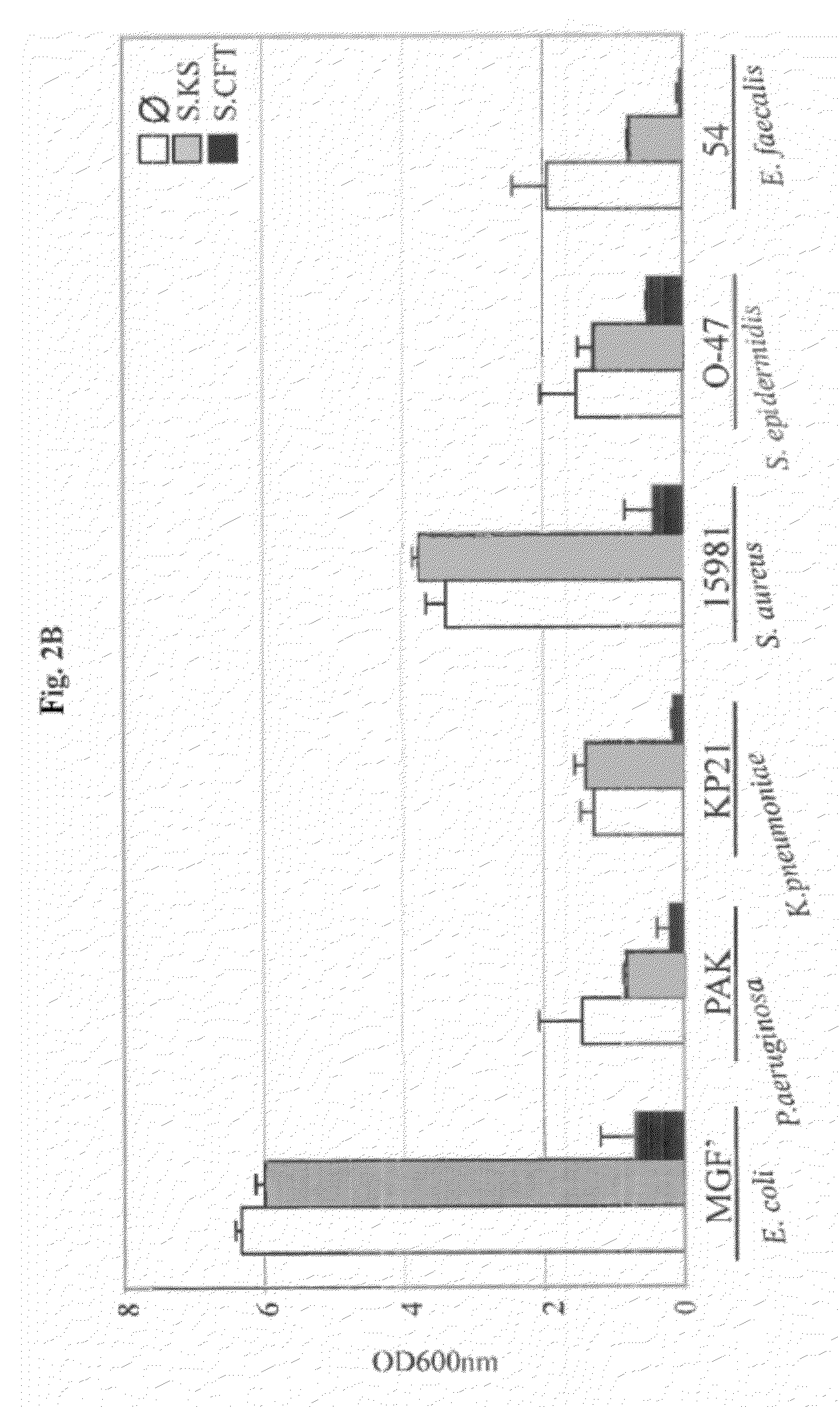
[0075]In order to study UPEC interactions within multicellular biofilm (Hall-Stoodley et al., 2004) bacterial communities, an in vitro mixed bacterial biofilm model in microfermentors was developed (Ghigo, 2001). Using this model, a 8 hours biofilm formed by the commensal strain of E. coli K12 MG1655 F′ was inoculated with different titers of the UPEC strain CFT073, and further cultivated for 24 hours. Upon increasing titers of CFT073, a strong reduction of the E. coli K12 MG1655 F′ biofilm development was observed, which was not observed when the commensal E. coli strain KS272 was used (FIG. 1A). This suggested that CFT073 could prevent MG1655 F′ biofilm formation either by direct contact or by secretion of an inhibitory molecule. To distinguish between these two possibilities, the supernatant of CFT073 stationary phase culture was filter-sterilized and its effect on E. coli biofilm formation was tested. In the presence of CFT073 supernata...
example 3
Correlation Between Anti-biofilm Activity and Type-II Capsule
[0077]To elucidate the genetic basis of the anti-biofilm effect, the supernatant activity of ca. 10,000 CFT073 random mariner transposon insertion mutants was tested. The inventors identified seven candidates impaired in their ability to inhibit MG1655 F′ biofilm formation. All these mutants mapped in genes involved in the expression of the group II capsular polysaccharide, the outermost bacterial cell surface structure (Whitfield and Roberts, 1999). Group II capsule displays a conserved modular genetic organization characterized by 3 functional regions (Roberts, 1996) (FIG. 3A). Region 1 (kpsFEDCUS) and region 3 (kpsMT) are conserved in all group II capsulated bacteria and encode proteins required for the ABC-dependent polysaccharide export. Region 2 is variable and encodes polysaccharide serotypes such as K1, K2 (CFT073), K5, K96 (Roberts, 1996). The R1, R2 or R3 region, or each individual kps gene was deleted and it was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com