Compositions and Methods for Treatment of Symptoms in Parkinson's Disease Patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
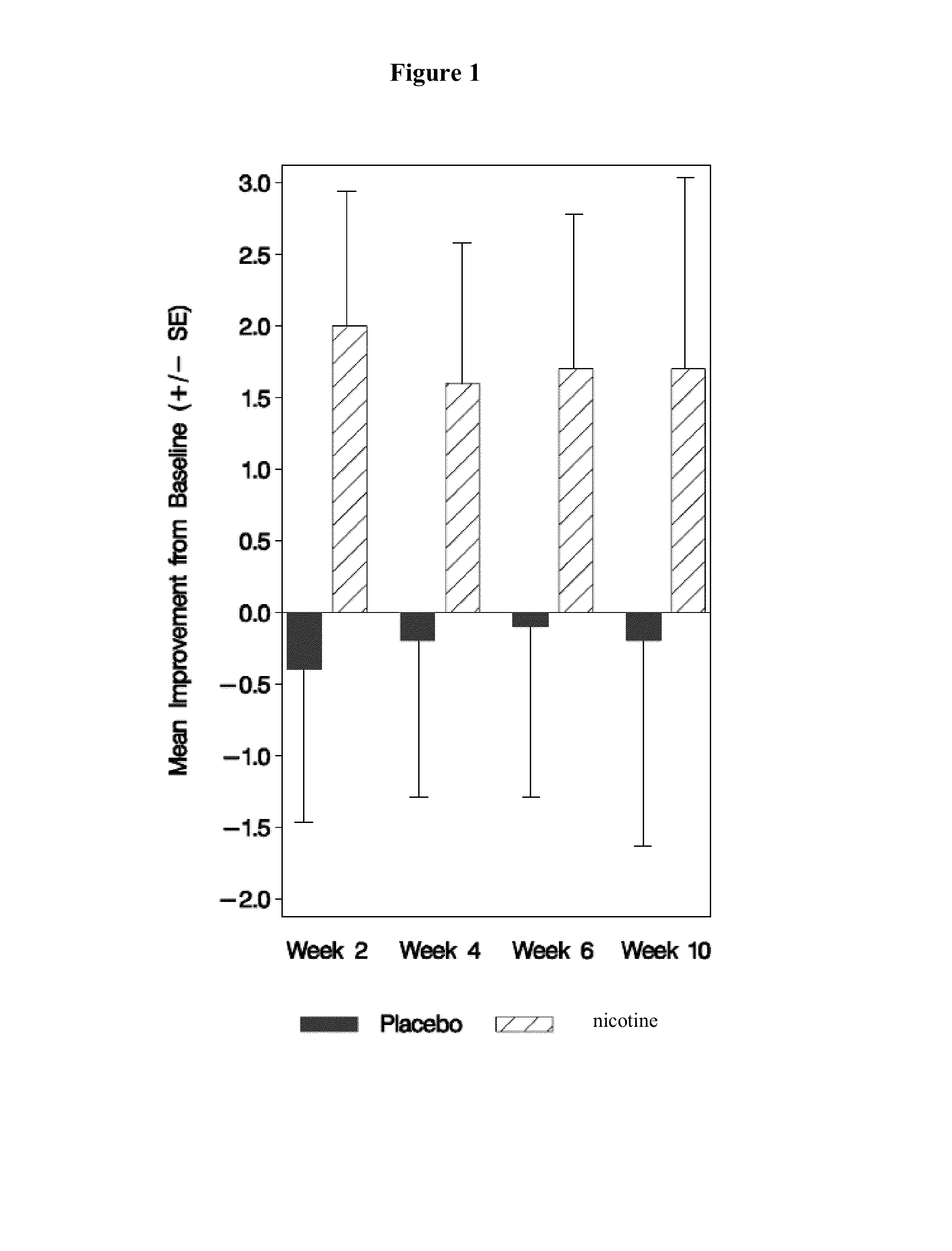
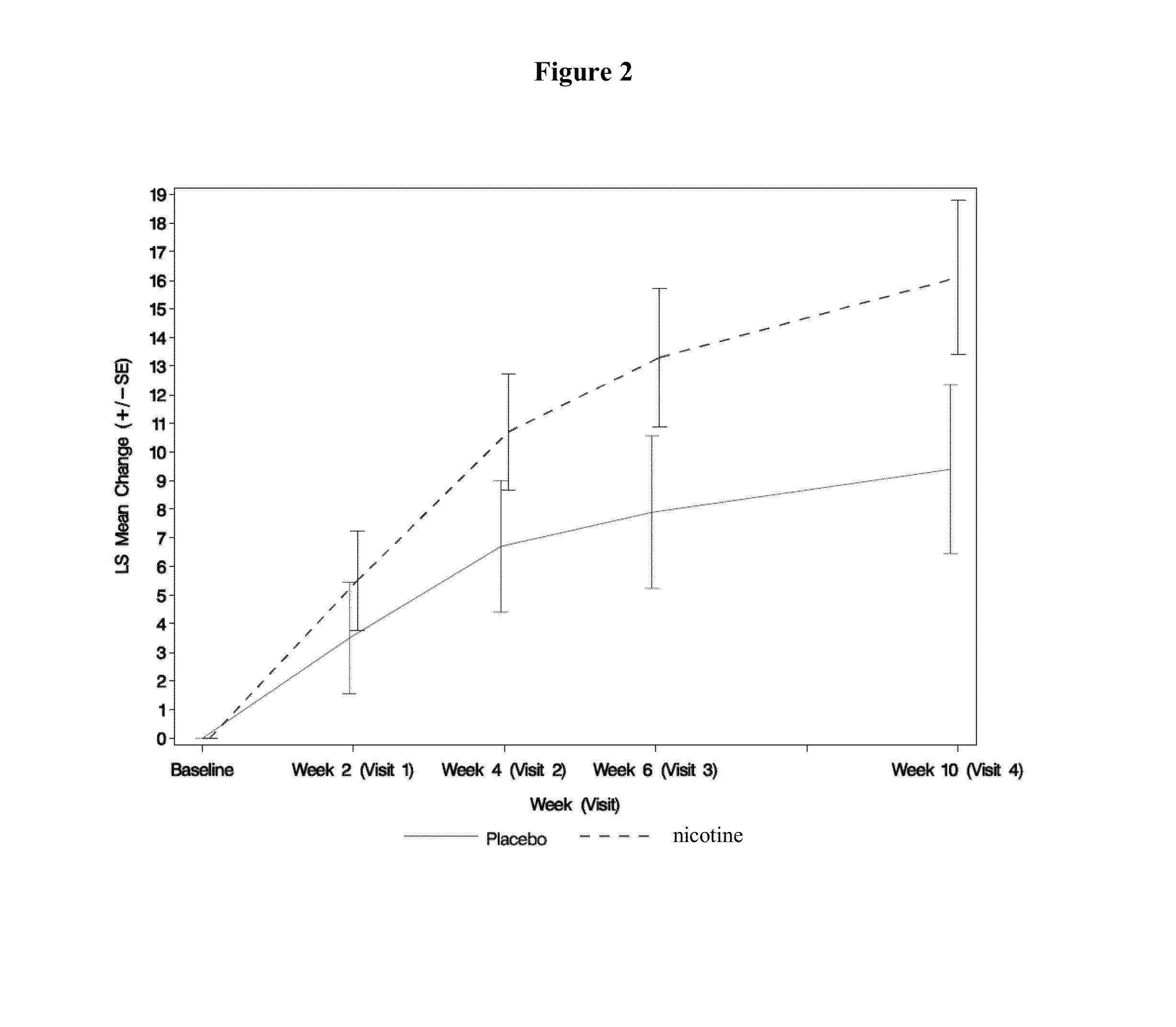
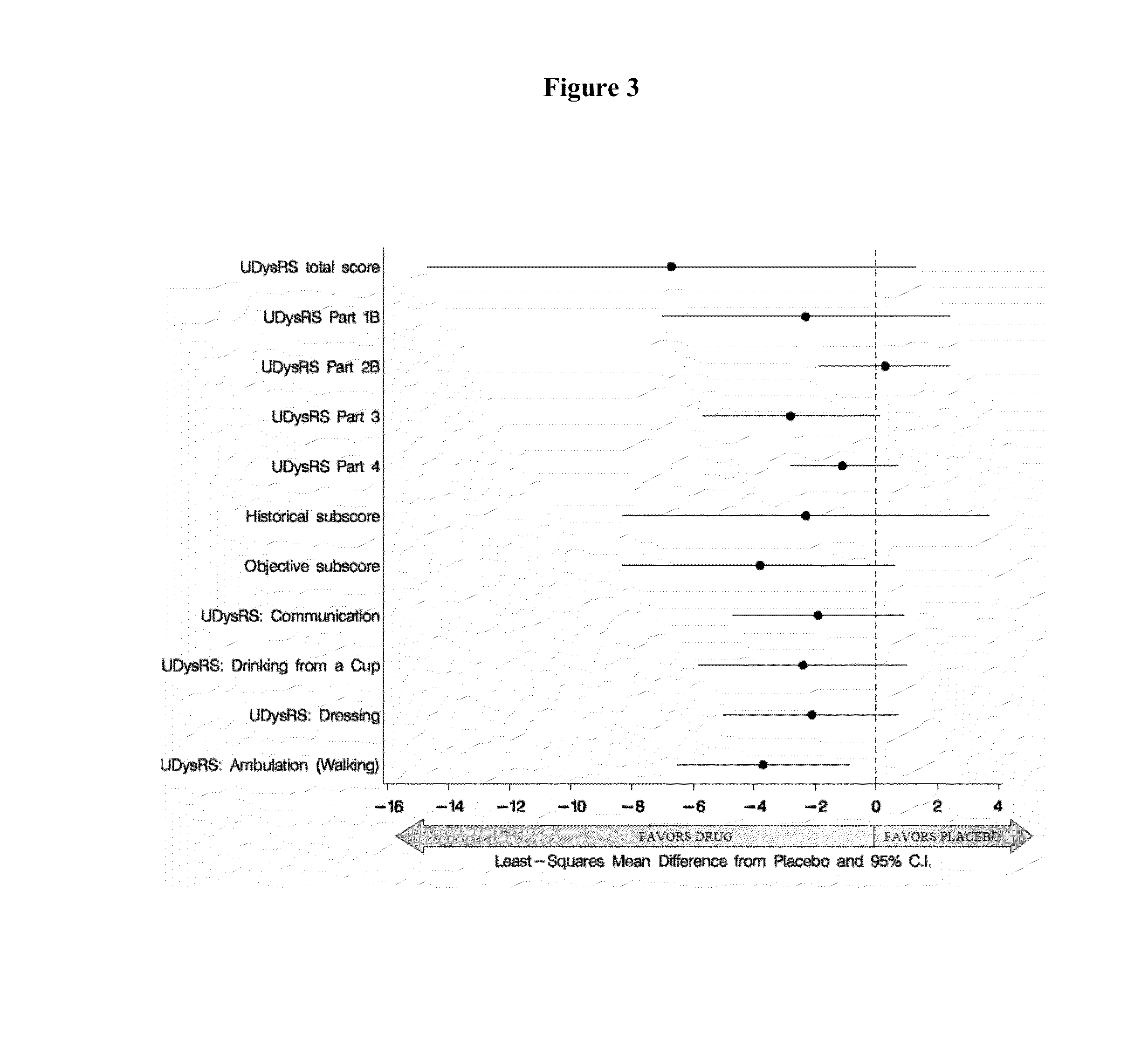
[0125]A total of 65 patients with idiopathic PD and LIDS were enrolled in a phase II study. Major entry criteria were as follows: 1) Hoehn and Yahr Stage II-IV while in “on” state, 2) had moderately to severely disabling LIDS>25% of waking day as determined by a rating of >2 on each of Questions 32 and 33 of the Unified Parkinson's Disease Rating Scale (UPDRS), 3) be on stable doses of levodopa and other medicines for PD for >30 days, 4) have a negative screening urine test for cotinine, and 5) not be a smoker or previous smoker or regular exposure to second-hand smoke. The study consisted of 3 phases: a treatment period of 10 weeks, a 9-day drug taper period, and a 5-day follow-up period. Subjects were randomly assigned to receive either nicotine or placebo (pbo). Dosing began at 1 mg q6hr and was escalated at 2-week intervals to 6 mg q6 hr (24 mg / day). All subjects were allowed to take ondansetron as rescue medication for the treatment of nausea and / or vomiting for t...
example 2
Formulation
[0136]Tablets are manufactured using a dry blend process, and hand made on a Carver ‘Auto C’ Press (Fred Carver, Inc., Indiana). The dry blend process consists of blending all of the ingredients in a plastic bag, and compressing into a 500 mg tablet (10 mg nicotine dose) using a 0.7086″.times.0.3937″ Mod Oval die (Natoli Engineering).
[0137]Tablets include nicotine, PEO Coagulant, Methocel K100M, and magnesium stearate. (PEO Coagulant=poly(ethylene oxide), grade PolyOx Coagulant, NF FP grade, manufactured by Union Carbide / Dow Chemical Company; Methocel K100M=hydroxypropylmethylcellulose, grade Methocel K100M, premium, manufactured by Dow Chemical Company; magnesium stearate, NF, supplied by Spectrum Chemical Company). Amounts of PEO Coagulant range from 10 to 90% by weight, amounts of Methocel K100M range from 10 to 90% by weight, and amounts of magnesium stearate range from 0 to 2% by weight.
[0138]The dissolution is determined in USP apparatus I (40 mesh baskets), 100 rpm...
example 3
Formulation
[0139]Example 2 is repeated with the percentage by weight of inactives as (i) 50% PEO Coagulant, 49% Methocel K100M, and 1% magnesium stearate; (ii) 89% PEO Coagulant, 10% Methocel K100M, and 1% magnesium stearate; and (iii) 10% PEO Coagulant, 89% Methocel K100M, and 1% magnesium stearate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com