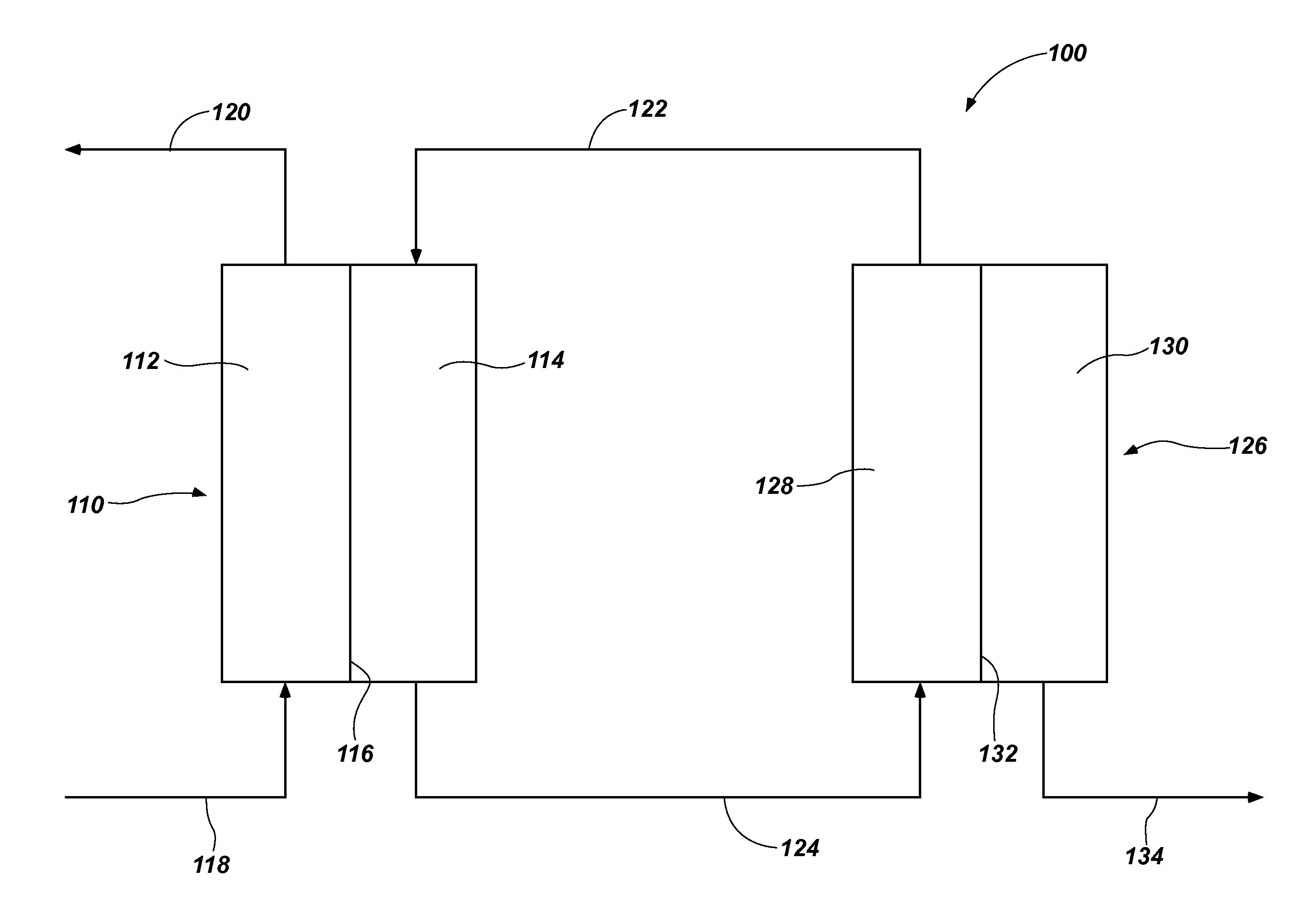
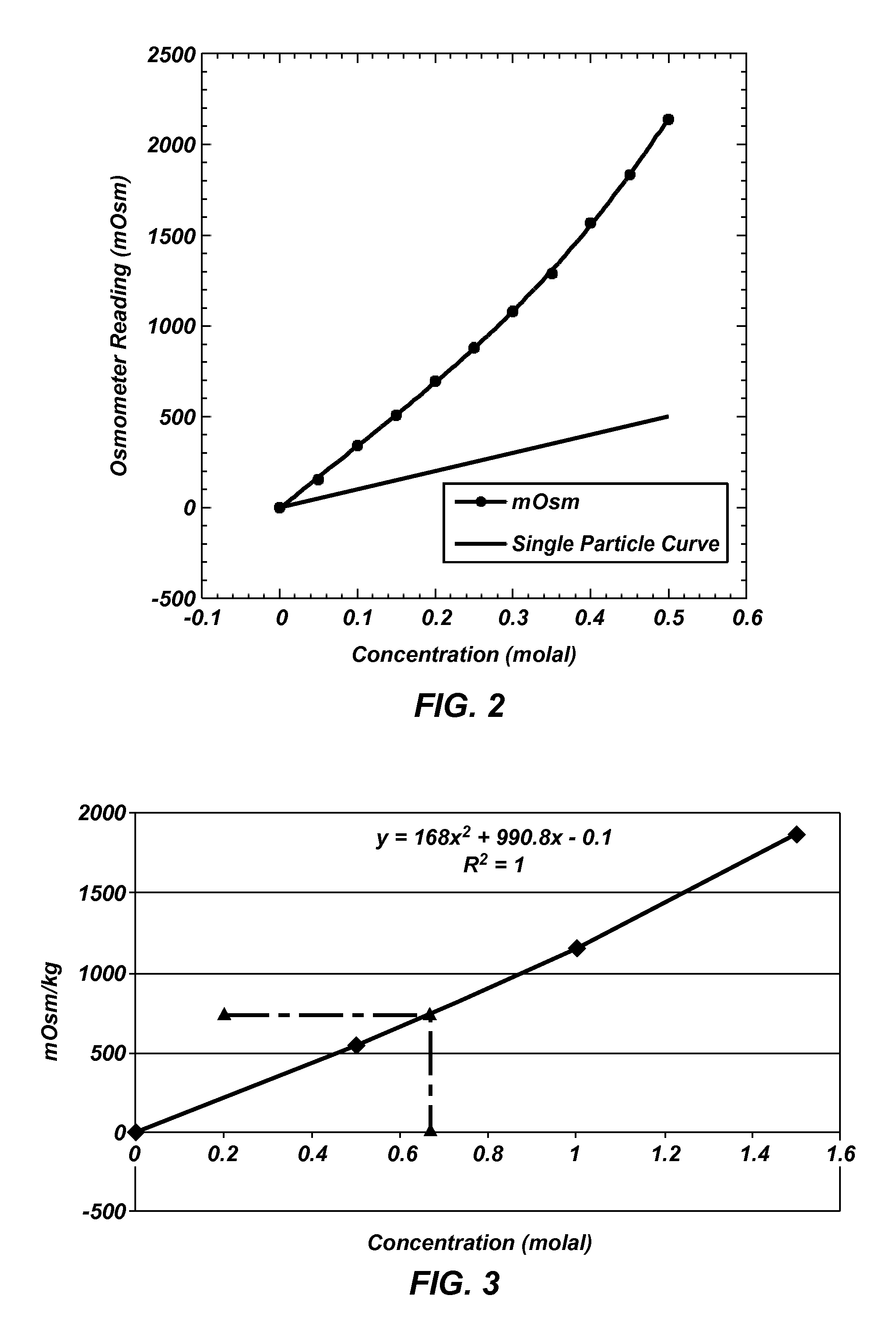
Draw solutes, methods of forming draw solutes, and methods of using draw solutes to treat an aqueous liquid
a draw solute and aqueous liquid technology, applied in the field of draw solutes, methods of forming draw solutes, and methods of using draw solutes to treat an aqueous liquid, can solve the problems of exacerbated membrane fouling by inorganic and organic molecules, substantial pressure to overcome the osmotic pressure of contaminated water sources, and significant investment in equipment and substantial ongoing energy costs. , to achieve the effect of less costly, greater osmotic pressur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis and Isolation of hexa(4-ethylcarboxylatophenoxy)cyclotriphosphazene
[0065]To a three neck round bottom flask was added a mechanical stirrer, a nitrogen purge, and a condenser. The flask was charged with approximately 300 ml of anhydrous tetrahydrofuran (THF). Sodium hydride (7.5 g, 326 mmol) was added slowly, followed by 4-ethylcarboxylatophenol (57.3 g, 345 mmol) dissolved in anhydrous 1,4-dioxane (˜300 ml). The resulting mixture reacted vigorously and was stirred for one hour to ensure completion. To this solution was added a solution of hexachlorocyclotriphosphazene (10 g, 29 mmol) in anhydrous 1,4-dioxane (50 ml). The reaction was heated to reflux and THF was removed using a Dean-Stark trap until the boiling mixture reached 85° C. The reaction mixture was stirred for 43 hours under a nitrogen purge. Reaction progress was monitored by P-31 Nuclear Magnetic Resonance (NMR) spectrometry. Isolation of the compound was accomplished by filtration of the cooled reaction soluti...
example 2
Formation of hexa(4-carboxyphenoxy)cyclotriphosphazene from hexa(4-ethylcarboxylatophenoxy)cyclotriphosphazene
[0066]In an oven dried 500 ml round bottom flask was added anhydrous THF (300 ml) and potassium tert-butoxide (16 g, 143 mmol). The resulting solution was cooled to 0° C. in a water ice bath. In a separate flask, hexa(4-ethylcarboxylatophenoxy)cyclotriphosphazene (3 g, 2.1 mmol) was dissolved in anhydrous THF (20 ml). The solution was added to the base solution and the resulting mixture was allowed to come to room temperature and was stirred overnight. After stirring, the resulting mixture was poured into water (˜1 L) and the pH was adjusted to approximately 3 with concentrated hydrochloric acid where a fine white precipitate was observed to form. Centrifugation of the precipitation solution yielded 2.1 g of a fine white powder for an 80% yield.
example 3
Formation of Sodium hexa(4-carboxylatophenoxy)cyclotriphosphazene from hexa(4-carboxyphenoxy)cyclotriphosphazene
[0067]Neutralization of the acidic functionality of the product of Example 2 was performed carefully to ensure excess base was not added. A solution of sodium hydroxide was prepared and standardized against dry potassium hydrogen phthalate (KHP) to yield a concentration of 0.177 M Na. Using this solution, a sample of hexa(4-carboxyphenoxy)cyclotriphosphazene (5 g, 4 mmol) was neutralized by suspending the phosphazene in water (˜20 ml). The base was added using a buret. To assure that no excess sodium hydroxide remained, dry hexa(4-carboxyphenoxy)cyclotriphosphazene (˜100 mg) was added incrementally to a final pH of 8. Once complete, the water was removed by rotary evaporation to give sodium hexa(4-carboxylatophenoxy)cyclotriphosphazene as a white powder in quantitative yield.
PUM
| Property | Measurement | Unit |
|---|---|---|
| osmotic pressure | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com