Conjugate of a polymer, an Anti-angiogenesis agent and a targeting moiety, and uses thereof in the treatment of bone related angiogenesis conditions
a technology of angiogenesis agent and polymer, which is applied in the field of conjugation of polymer and anti-angiogenesis agent, can solve the problems of restricting their use, and achieve the effect of low polydispersity and high load of alendrona
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of HPMA Copolymer-ALN-TNP-470 Conjugate
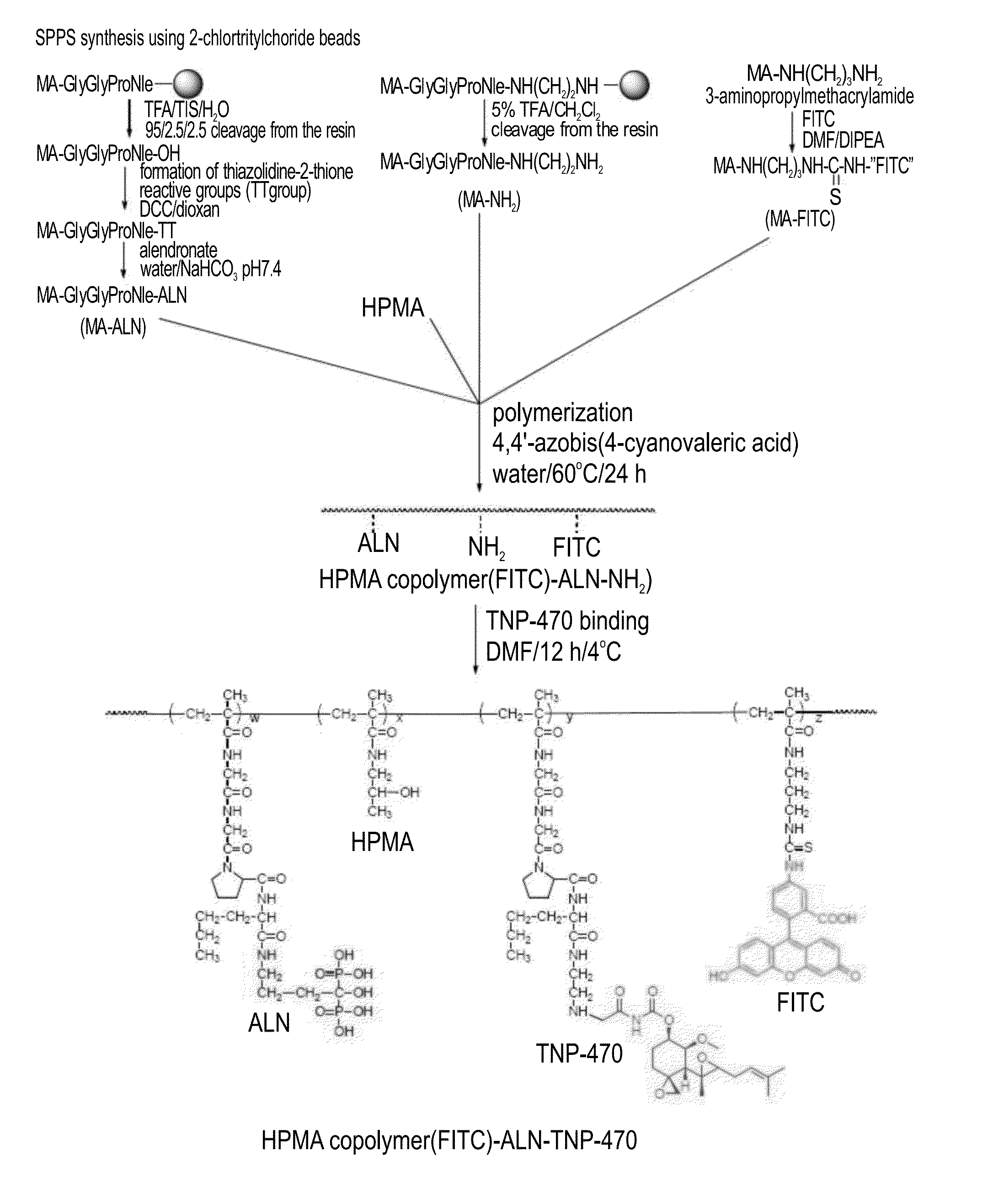
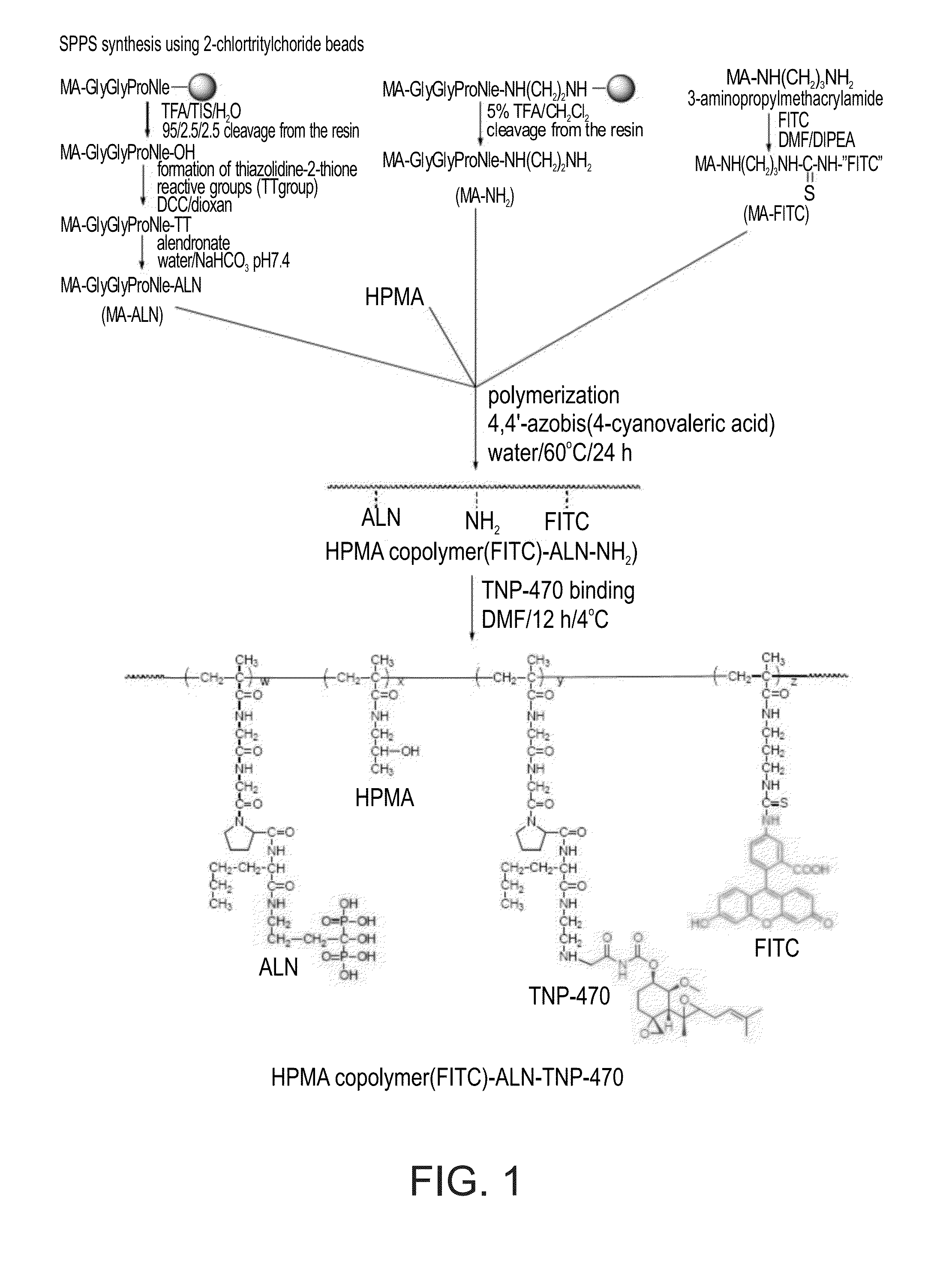
[0494]The general synthesis of a HPMA-ALN-TNP-470 conjugate according to some embodiments of the present invention is depicted in FIG. 1.
[0495]HPMA-Gly-Gly-Pro-Nle-ALN-TNP-470 (SEQ ID NO: 12) conjugate was prepared in a two-step synthesis. First, an intermediate was synthesized by copolymerization of HPMA, ALN-methacrylamide monomer (MA-Gly-Gly-Pro-Nle-ALN; (SEQ ID NO: 8), and amino group-containing methacrylamide monomer (MA-Gly-Gly-Pro-Nle-ethylenediamine; SEQ ID NO: 13). Optionally, for the evaluation of intracellular trafficking, a polymerizable derivative of fluorescein isothiocyanate (FITC), N-methacryloylaminopropyl fluorescein thiourea (MA-FITC), was added to the monomer mixture. Next, TNP-470 was linked to free amino groups by nucleophilic substitution of the terminal chlorine of TNP-470.
[0496]Synthesis of ALN-containing monomer (MA-Gly-Gly-Pro-Nle-ALN ; SEQ ID NO: 8): MA-Gly-Gly-Pro-Nle (SEQ ID NO: 7) was synthesized by soli...
example 2
Effect of Combination Treatment of ALN and TNP-470 on Proliferation of Endothelial Cells In Vitro
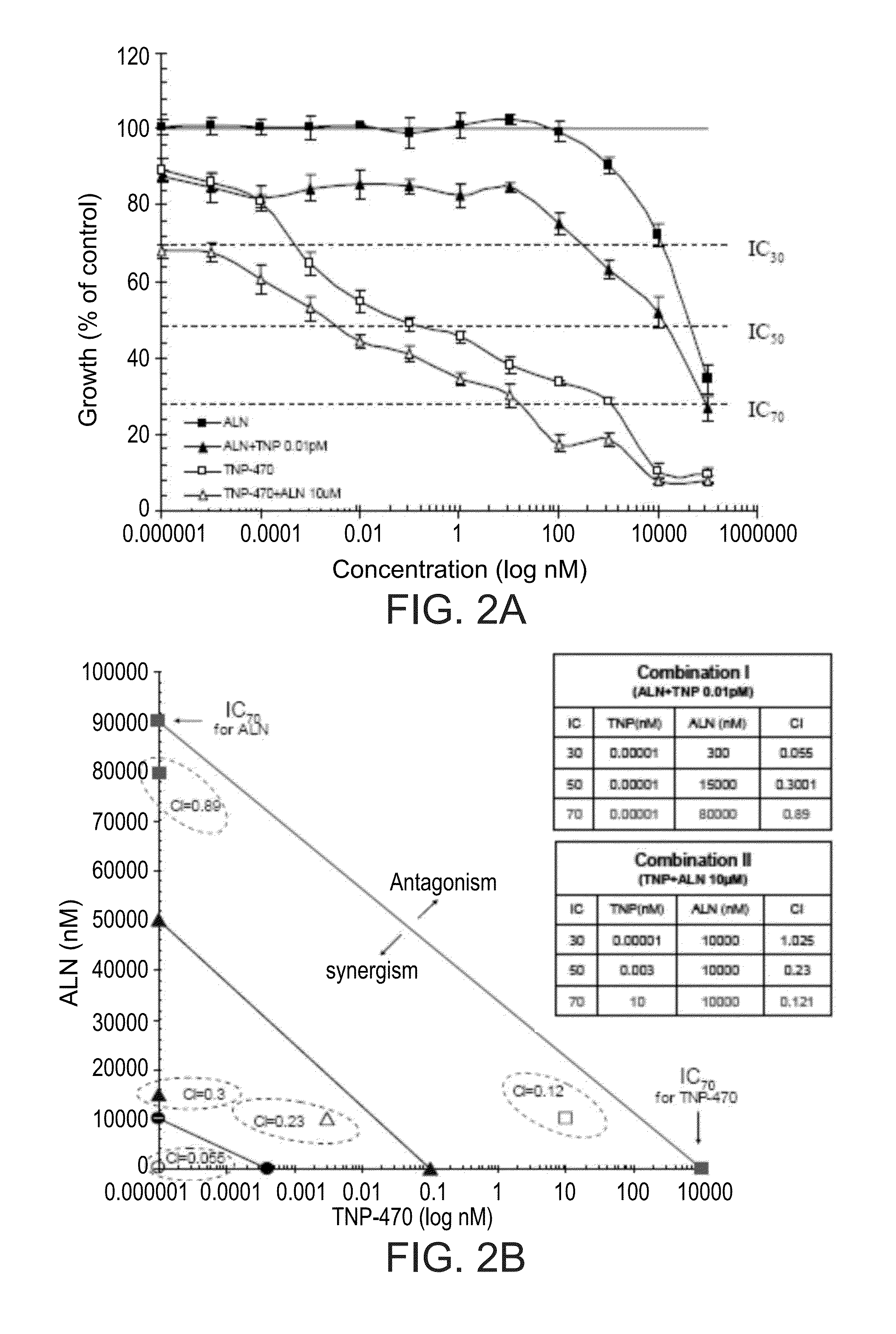
[0515]Prior to conjugation of ALN and TNP-470 to HPMA copolymer backbone, the nature of the inhibitory effect of ALN and TNP-470 as a combined therapy on endothelial cells proliferation in vitro was evaluated. HUVEC were challenged with free or combined ALN and TNP-470. The results, presented in FIG. 2A show that combination treatments of ALN and TNP-470 decreased the IC's of the drugs as single treatments. ALN inhibited cell proliferation at inhibitory concentration IC30, 50, 70 of 10, 50, 90 μM, respectively, and TNP-470 inhibited cell proliferation at IC30, 50, 70 of 0.00025, 0.1, 1000 nM, respectively. Combination treatments I (serial concentrations of ALN and TNP-470 0.01 pM) and II (serial concentrations of TNP-470 and ALN 10 μM) inhibited HUVEC proliferation at IC30, 50, 70 of 0.2, 10, 30 μM and 0.00001, 0.004, 40 nM, respectively.
[0516]Combination index (CI)-isobologram equation ...
example 3
Binding of a HPMA -ALN-TNP-470 Conjugate to Bone Mineral Hydroxyapatite
[0517]One of the main characteristics of ALN besides its anti-angiogenic and antitumor activities is its pharmacokinetic profile which exhibits a strong affinity to bone mineral under physiological conditions. To determine if the activity of ALN is retained following polymer-conjugation, the affinity of the conjugate to bone mineral was evaluated by in vitro hydroxyapitate binding assay and FPLC analysis using Hitrap desalting column. As shown in FIG. 3B, unbound conjugates eluted as a single peak with a retention time of 1.9 minutes. AUC decreased in correlation with hydroxyapitate incubation time. Following 2 minutes of incubation with hydroxyapitate, 50% of the HPMA copolymer ALN-TNP-470 conjugate in the solution was bound to hydroxyapitate (see, FIG. 3C). This rapid binding rate to hydroxyapitate decreased after 10 minutes and finally reached a plateau after 175 minutes of incubation time with 92% of the HPMA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com