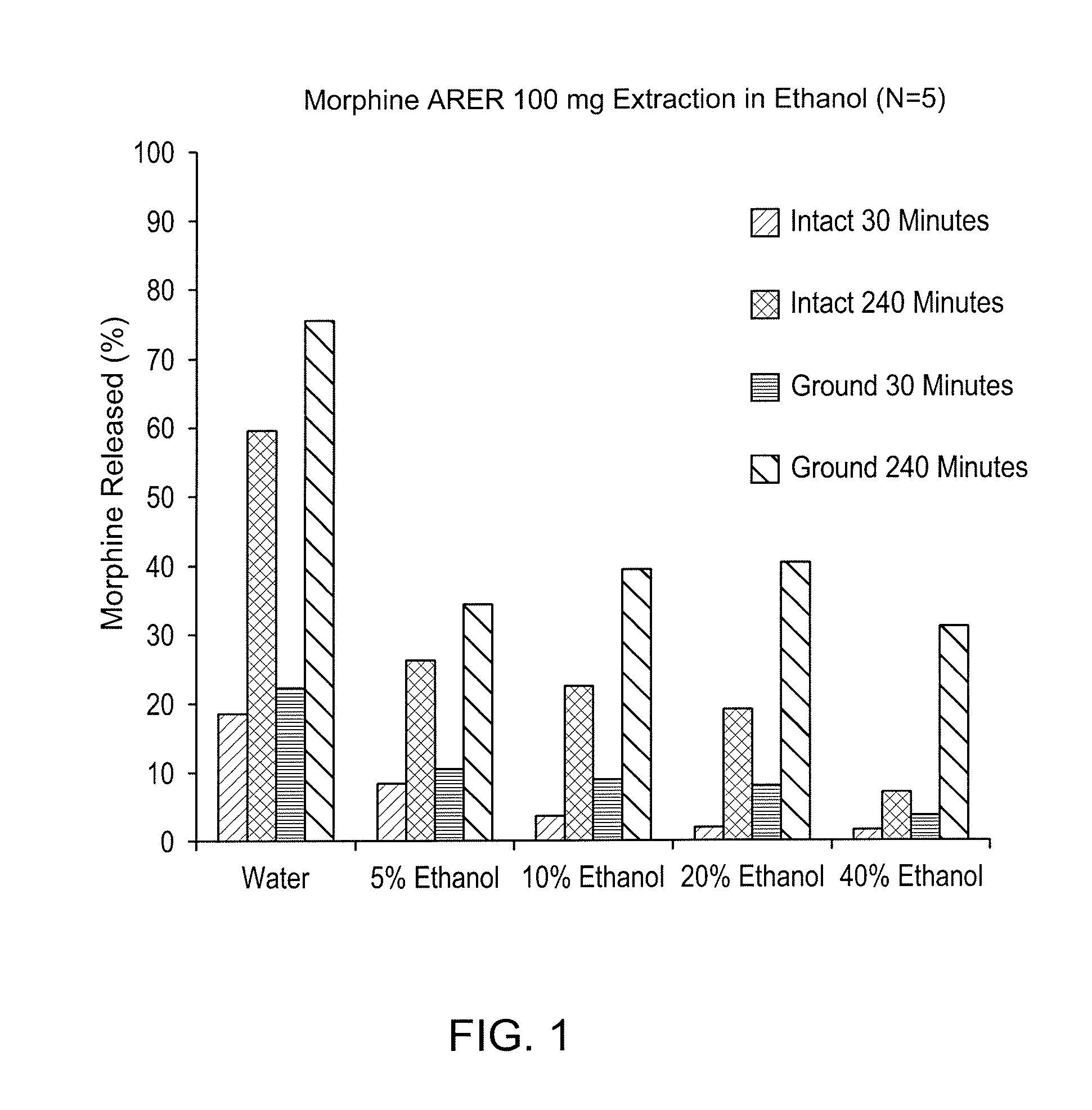
Abuse deterrent compositions and methods of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0111]The following formulations were tested:
mg / tab(approximate amounts)IngredientTest ATest BPresent inBinder(s)300300Tablet CoreFiller(s)5050Tablet CoreGlidant55Tablet CoreLubricant1010Tablet CoreEudragit NE 30D67671st CoatingFiller25251st CoatingSurfactant0.250.251st CoatingGlidant551st CoatingEudragit NE30D12.512.52nd CoatingEudragit NM30D12.512.52nd CoatingOxycodone30302nd CoatinghydrochlorideSurfactant112nd CoatingGlidant222nd CoatingEUDRAGIT ® E100103rd CoatingETHOCEL ® 4533rd Coating(ethylcellulose)Plasticizer23rd CoatingLubricant53rd CoatingOpadry II film-coating10104th CoatingTOTAL TABLETabout 550 mgabout 530 mgWEIGHT
[0112]The following tables show the dissolution profile of TEST A tablets, which are tablets of the present invention; TEST B tablets and ROXICODONE® tablets, which are comparative tablets.
[0113]TABLE 1 shows the dissolution profile in acidic conditions (0.1 N Hydrochloric acid).
TABLE 1Comparative Dissolution Profile in 0.1N Hydrochloride acid; 500 mL;Paddles,...
example 2
[0115]The following coating formulations were tested, both in acidic medium (HCl) and neutral pH medium (deionized water):
Amount in mgIngredientsCoating 1Coating 2Coating 3Coating 4Coating 5Coating 6EUDRAGIT ® E10010.020.010.010.00.010.0DBS1.53.01.51.51.51.5Magnesium Stearate3.57.03.53.53.53.5ETHOCEL ® 450.00.02.03.03.05.0*Ethanol13527015316272180Total weight gain15.030.017.018.08.020.0Release in HClAcceptableAcceptableAcceptableAcceptableAcceptableNotacceptable;NLT 75%requiredafter 45minutesRelease In DI WaterVery fast; Very fast;ReleaseAcceptableVery fast; Acceptablenotnotmore thannotacceptableacceptable10% afteracceptable60 minutes;notacceptable*Evaporated during the process
Dissolution Data for the Above Examples in 0.1 N HCl and DI Water
[0116]
% Released for the above examples in 0.1N HCl and DI WaterTimeCoating 1Coating 2Coating 3Coating 4Coating 5Coating 6(min) HClWaterHClWater HClWaterHClWater HClWater HClWater5494057585322105051201075697975834641767513015918492929989229189260...
example 3
[0117]The following study was conducted to determine the relative bioavailability and abuse potential of equivalent doses of a crushed and intact formulation. The study was a randomized, double-blind study, wherein 26 subjects were tested. The following formulation was tested:
[0118]An extended release tablet formulation of the present invention containing 60 mg of morphine sulfate pentahydrate was tested.
[0119]The following mean exposures (AUC0-t) were observed after oral administration of a 60 mg intact tablet and intranasal administration of a ground 60 mg tablet.
MORPHINEM6GAUC (ng · h / mL)AUC (ng · h / mL)intranasaloralintranasaloraladministration ofadministrationadministration ofadministration ofground tabletof intact tabletground tabletintact tabletCmax24.0317.7249.41106.98AUC 0-t158.3132.86385.58830.12AUC 0-0.52.531.760.481.84AUC 0-110.176.963.8417.33AUC 0-231.421.530.8995.04AUC 0-8109.9685.64233.64537.79AUC 0-12130.18101.14294.23649.01AUC 0-24162.78132.92398.2830.29
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com