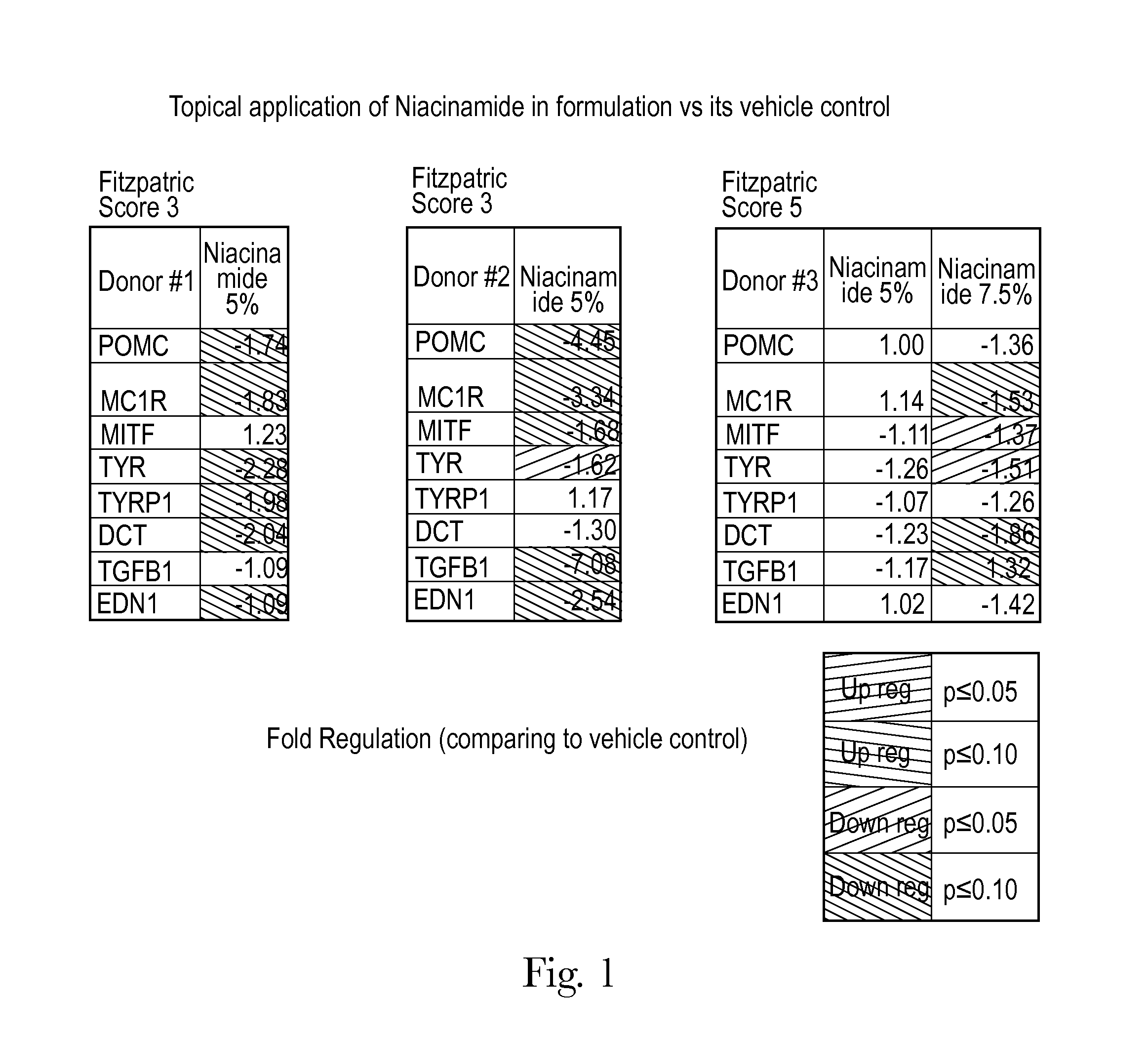
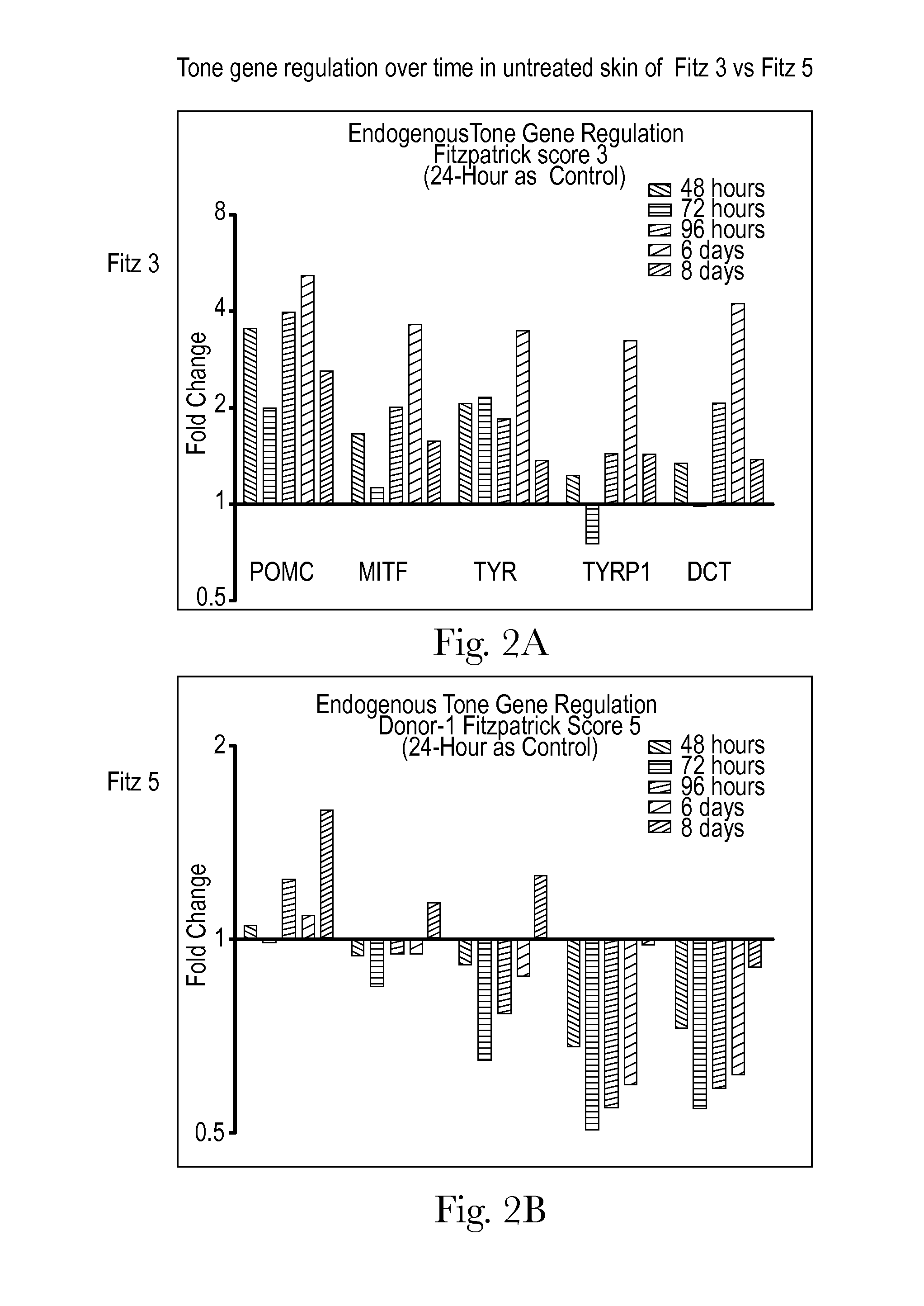
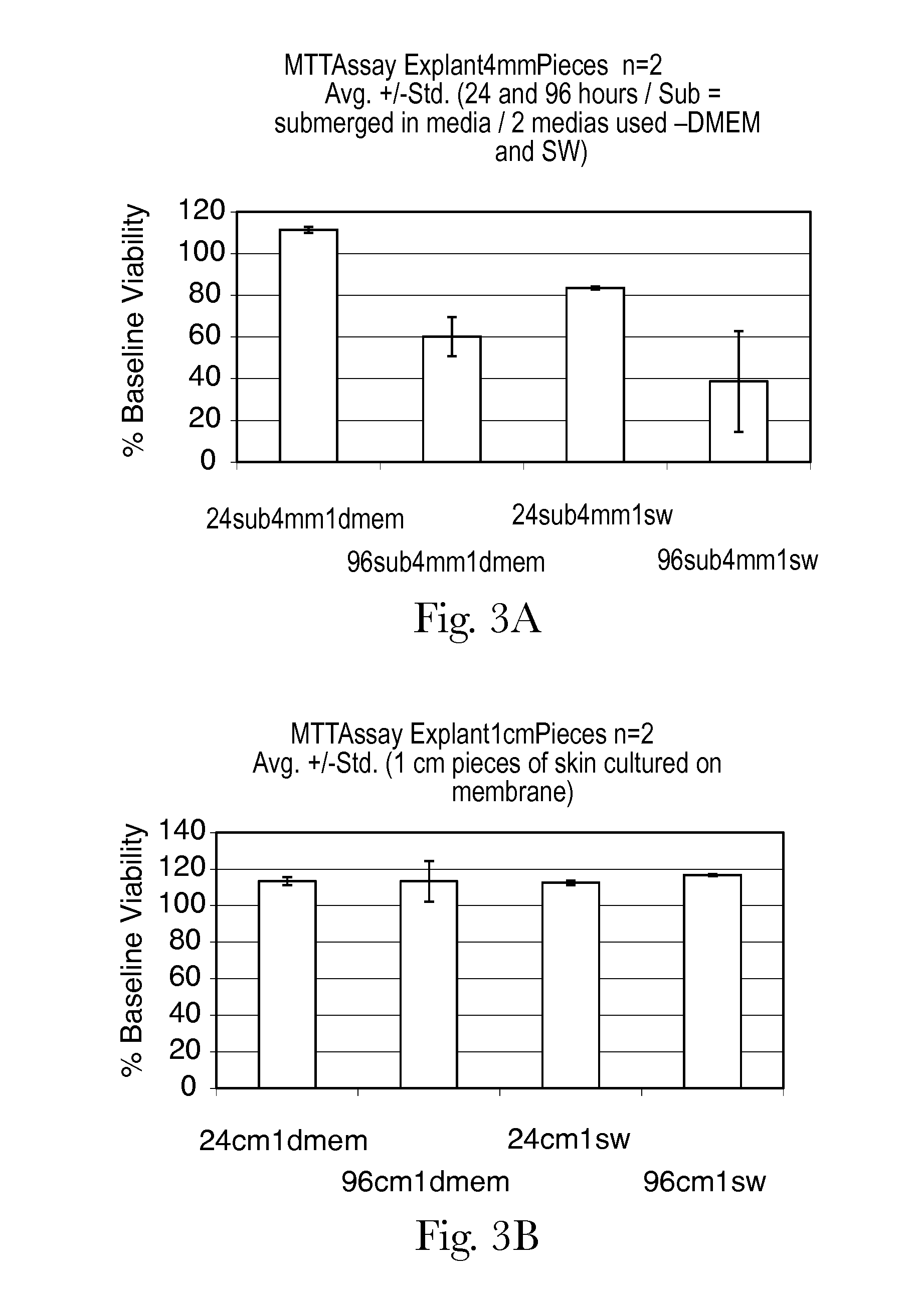
Human skin sample methods and models for validating hypotheses for mechanisms driving skin pigmentation
a skin pigmentation and human skin technology, applied in the field of human skin sample methods and models for validating hypotheses of mechanisms driving skin pigmentation, can solve the problems of inability to account for intrinsic intricate matrices of cells constantly interacting, easy to use single cell types, and difficult to generalize results to human skin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0106]Individual experiments generally comprise processing of human surgical waste skin used for related ex-vivo (explant) studies. An example embodiment is herein described below:
[0107]Materials for preparation included: Dulbecco's Modified Eagle Medium plus Glutamax, antibiotic and antimycotics, 1×phosphate buffer solution (PBS (-ca, -mg)), 150×15 mm sterile culture dishes, sterile gauze 4×4, disposable safety scalpels, 4 mm disposable biopsy punch, 6-well cell culture inserts, disinfected tweezers, cotton-Tipped Applicators, 6-well culture plates, ruled, disinfected cutting mat (12×12), scraper handle, tissue freezing medium, biopsy cassettes, frozen tissue freezing vessels, 10% formalin, shipping containers for fixed tissue, and biohazard / sharps containers.
[0108]Skin preparation involved preparation of media in advance of the arrival of skin (3×media including 500 ml DMEM plus 15 ml of antibiotic and antimycotics; The 3× referred to 3-fold levels of antibiotics / a...
example 2
Culturing
[0111]An example embodiment of culturing using ex-vivo skin is herein described below:
[0112]Culturing involved setting up culture plates: putting 2.5 ml of media or treatment into each well of a 6-well tissue plate, adding one Millicell culture insert to each well, and putting 100 μl of 1×DMEM on the center of each insert membrane. Squares were selected randomly placed into each of the inserts (one piece of skin per insert), on top of the media on the membrane. The plates were stored at either 33° C. (though an additional non-limiting example would be 37° C. depending on the experiment endpoint). As a control, baseline tissue measurements were collected from excess waste (cryogenics were used for snap freezing). Baseline biopsy collection involved two 4 mm punches for MTT; One 4 mm punch was snap frozen in OCT for fresh histology; One 4 mm punch was fixed in 10% formalin and sent out for paraffin embedding. PCR baseline was also be taken with six 4 mm punches snap frozen in...
example 3
mRNA Processing, Nanodrop-Quantification and Purity Assessment, RNA Quantification Gel Protocol, cDNA Synthesis. and PCR Setup mRNA Processing
[0119]mRNA was processed for the ex-vivo skin methods. An example embodiment is herein described below:
[0120]For cryopreparation and extraction the following equipment was obtained and prepared: 2 ml round bottom tubes were prepared with 1 ml of TRIZOL as was a 5 mm stainless steel bead for each sample; a dry ice bucket was obtained and liquid nitrogen was placed in the bucket. Biopsy punches were maintained on dry ice, and samples remained frozen. Cryobags and covaris cryoprep were used in freeze fracturing of samples. To freeze fracture the samples, biopsies were placed in a cryo bag (2 per sample) and dipped in liquid nitrogen, then placed in cryoprep (setting 4). The sample bag was taken out and dipped back into nitrogen. A flattened disk was removed and placed into corresponding 2 ml tubes with TRIZOL. The disk was kept frozen in the TRIZ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com