Inhibitor of trpm-4 ion channel for treating or preventing neurodegeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
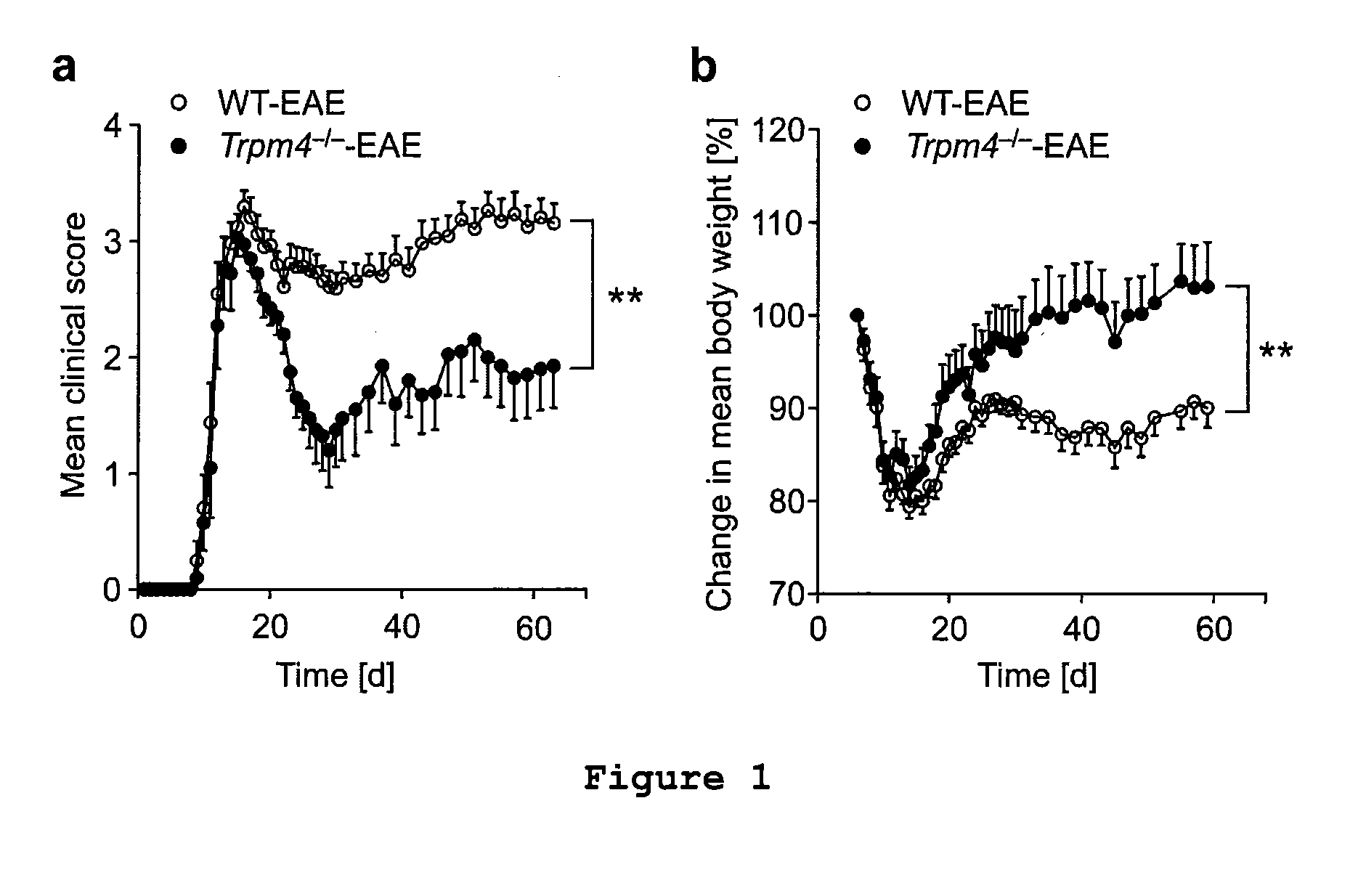
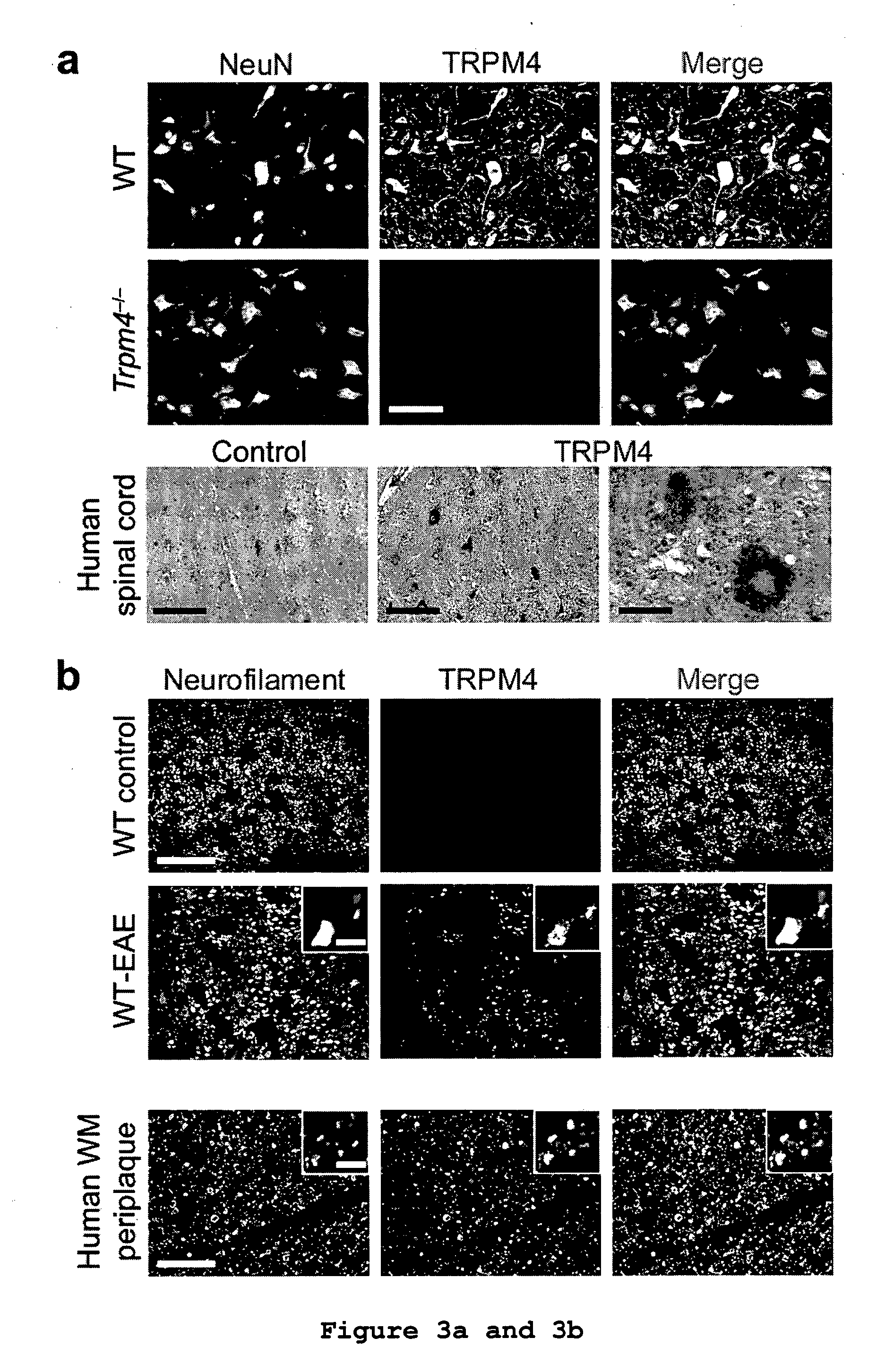
TRPM4 Deficiency Ameliorates Experimental Autoimmune Encephalomyelitis (EAE)
[0062]In order to investigate whether TRPM4 modulates the pathogenesis of EAE, knockout mice with a dysfunctional Trpm4 gene (Trpm4− / −) and wild-type (WT) mice were immunized with the myelin oligodendrocyte glycoprotein peptide 35-55 (MOG35-55) in order to induce EAE in these animals. The sequence of the MOG35-55 peptide used for immunization is shown in SEQ ID NO:3. Briefly, C57BL / 6 Trpm4− / − mice (Vennekens et al. (2007), Nat Immunol. 8:312-320) and Trpm4+ / + littermates (referred to as WT controls) were immunized subcutaneously with 200 μg / mouse MOG35-55 in complete Freund's adjuvant (Sigma-Aldrich) containing 4 mg / ml Mycobacterium tuberculosis (H37Ra, Difco). In addition, 200 ng pertussis toxin (Calbiochem) was injected intravenously on the day of immunization and 48 h later. The mice were sex and age (6-10 weeks) matched and were scored for clinical signs every day over a period of 60 days by the followin...
example 2
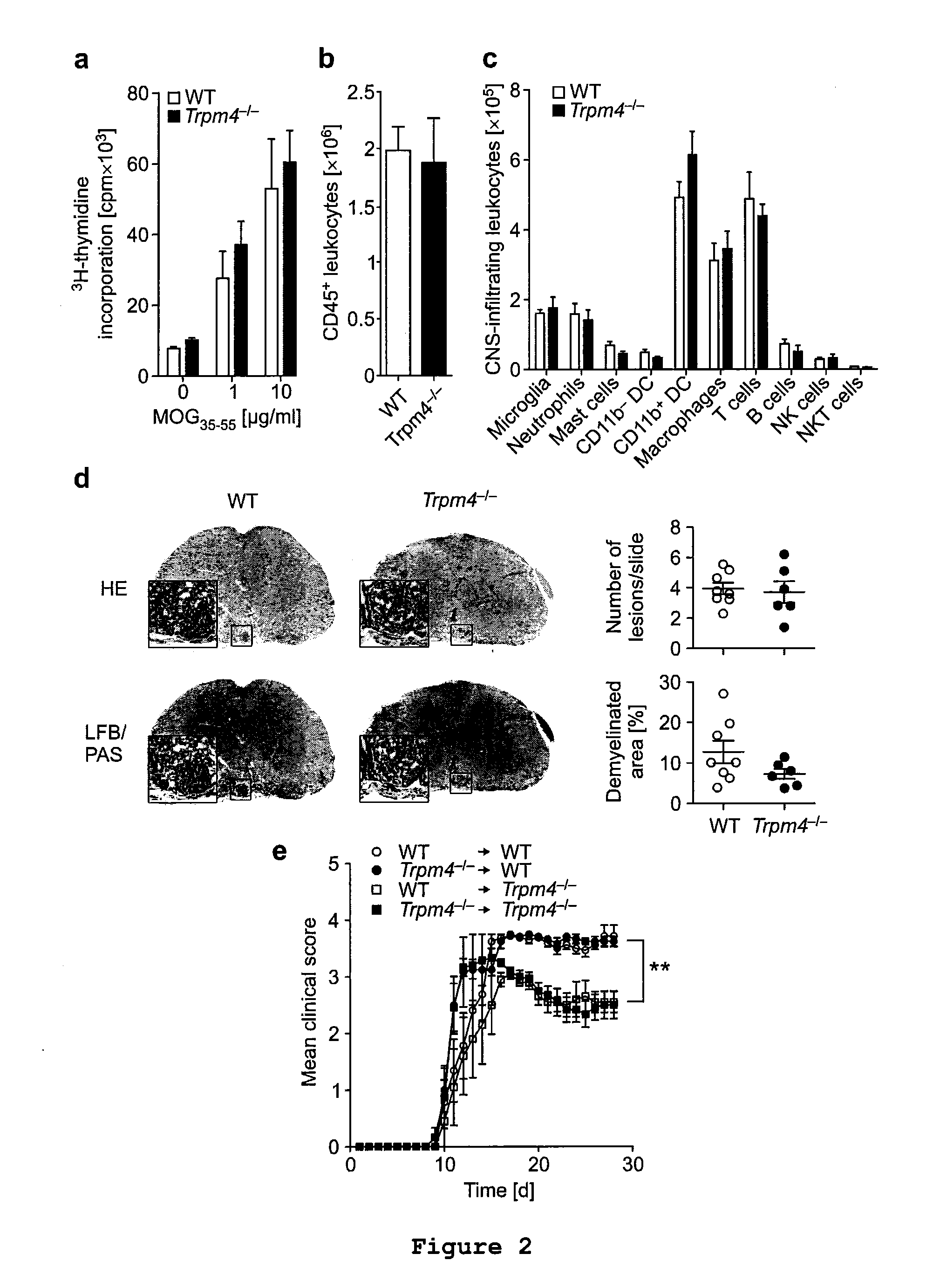
Analysis of Immune Cell Activation by Detecting Incorporation of [methyl-3H]-thymidine
[0065]Since EAE is an autoimmune disease and TRPM4 was previously shown to fulfil functions in immune cell activation and migration, it was first examined whether the protective phenotype in Trpm4− / − could be explained by altered immune cell activation or infiltration. For this purpose, C57BL / 6 Trpm4− / − mice and WT controls were treated as described in Example 1. For assessing T-cell proliferation, single cell suspensions from draining lymph nodes and spleens were prepared 15 days after immunization from WT EAE (n=6) and Trpm4− / − EAE (n=6) mice by homogenizing the tissues through a 40 μm cell strainer (BD Biosciences). Cells were sedimented by centrifugation (300×g, 7 min, 4° C.) and splenic red blood cells were lysed in red blood cell lysis buffer for 7 min at 4° C. Cells were washed once in PBS and resuspended in buffer. The lymph node cells obtained from the immunized animals were cultured in 96...
example 3
Analysis of CNS Infiltrates by Flow Cytometry
[0068]Since the protective phenotype observed in Example 1 could not be explained by altered immune cell activation, CNS (brain and spinal cord) infiltrates were analyzed by flow cytometry. For the isolation of CNS-infiltrating immune cells, WT EAE (n=4) and Trpm4− / − EAE (n=3) mice were sacrificed by CO2 inhalation and immediately transcardially perfused with ice-cold PBS. Brain and spinal cord were removed, minced with blades and digested in collagenase / DNAseI solution (Roche) for 45 min at 37° C. Tissue was triturated through a 40 μm cell strainer. The homogenized tissue was washed in PBS (300× g, 10 min, and 4° C.). Immune cells including microglia were separated from myelin, other glia and neuronal cells by centrifugation over a discontinuous percoll (GE Healthcare) gradient. The homogenized tissue was resuspended in 30% isotonic percoll, transferred into a 15 ml Falcon tube and 78% isotonic percoll was layered underneath. The gradien...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dimensionless property | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
| Body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com