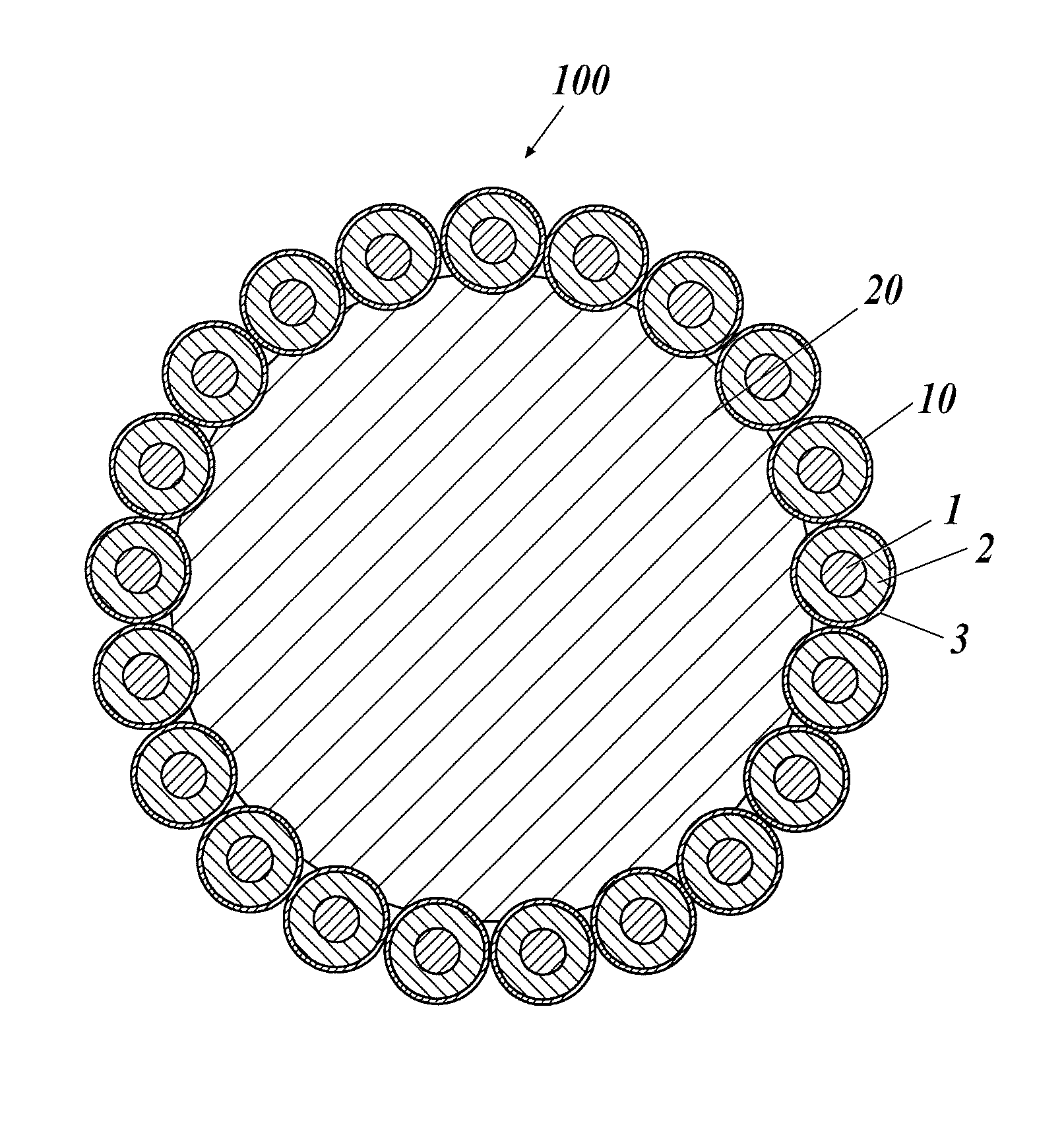
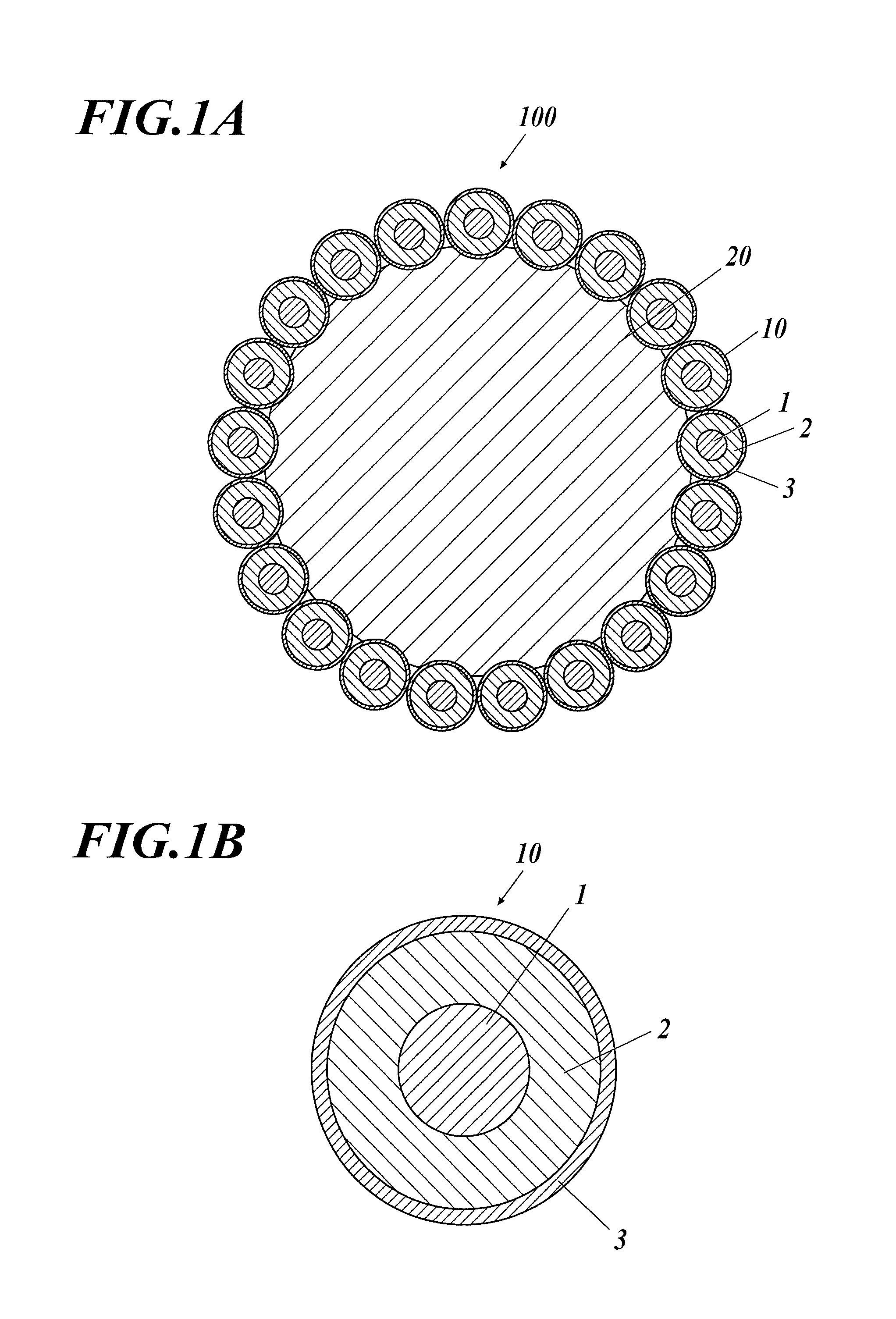
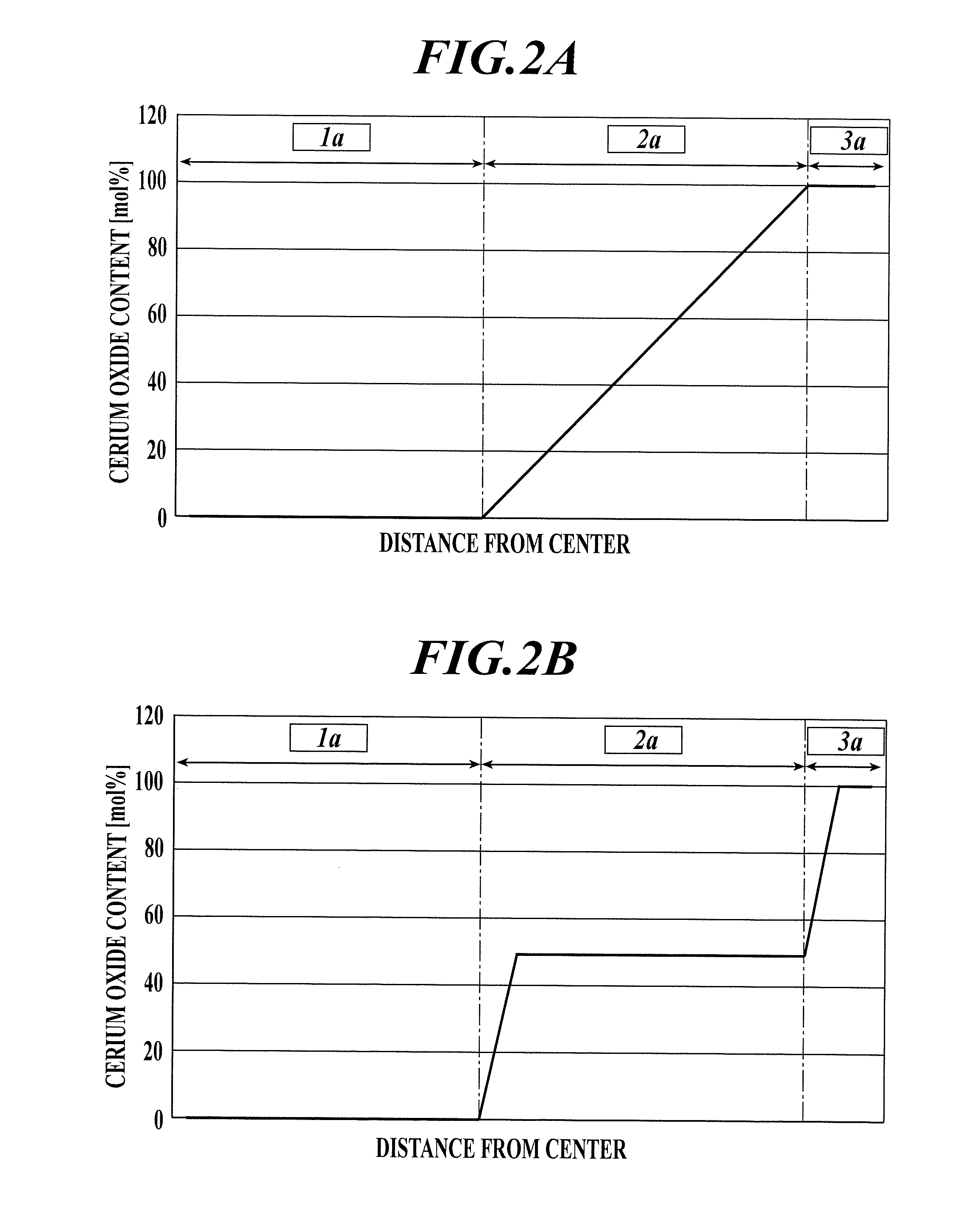
Polishing Material Composition And Production Method Therefor
a technology of polishing material and composition, which is applied in the direction of chemistry apparatus and processes, other chemical processes, metal-working apparatus, etc., can solve the problems of high specific gravity of chrome oxide, scratches and scrapes on polished workpieces, and unfavorable dispersion, so as to reduce the surface roughness of polished surfaces, improve the polishing rate and durability, and barely scratch workpieces
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Abrasive Material 1
[0082]Step 1. Urea was added to 10 L of aqueous yttrium nitrate (0.01 mol / L) solution into a urea concentration of 0.20 mol / L. The mixture was sufficiently stirred and was then heated at 90° C. for 1 hour with stirring.
[0083]Step 2. A pre-prepared mixture of 300 mL of aqueous yttrium nitrate (0.08 mol / L) solution and 300 mL of aqueous cerium nitrate (0.32 mol / L) solution was added to the dispersion prepared in Step 1 at an addition rate of 10 mL / min with heating at 90° C. and stirring.
[0084]Step 3. 50 mL of aqueous cerium nitrate (0.4 mol / L) solution was added to the dispersion prepared in Step 2 at an addition rate of 10 mL / min with heating at 90° C. and stirring.
[0085]Step 4. The abrasive material precursor precipitated from the dispersion prepared in Step 3 was collected with a membrane filter and was fired at 600° C. to yield inorganic abrasive particles.
[0086]Step 5. To 600 mL of toluene were added 36 g of the inorganic abrasive particles produced in Step 4 a...
example 2
Abrasive Material 2
[0090]Step 1. Urea was added to 10 L of aqueous yttrium nitrate (0.01 mol / L) solution into a urea concentration of 0.20 mol / L. The mixture was sufficiently stirred and was then heated at 90° C. for 1 hour with stirring.
[0091]Step 2. A pre-prepared mixture of 300 mL of aqueous yttrium nitrate (0.08 mol / L) solution and 300 mL of aqueous cerium nitrate (0.32 mol / L) solution was added to the dispersion prepared in Step 1 at an addition rate of 10 mL / min with heating at 90° C. and stirring.
[0092]Step 3. 50 mL of aqueous cerium nitrate (0.4 mol / L) solution was added to the dispersion prepared in Step 2 at an addition rate of 10 mL / min with heating at 90° C. and stirring.
[0093]Step 4. The abrasive material precursor precipitated from the dispersion prepared in Step 3 was collected with a membrane filter and was fired at 600° C. to yield inorganic abrasive particles.
[0094]Step 5. One gram of azobisisobutyronitrile was added to a mixture of 50 mL of styrene and 50 mL of et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com