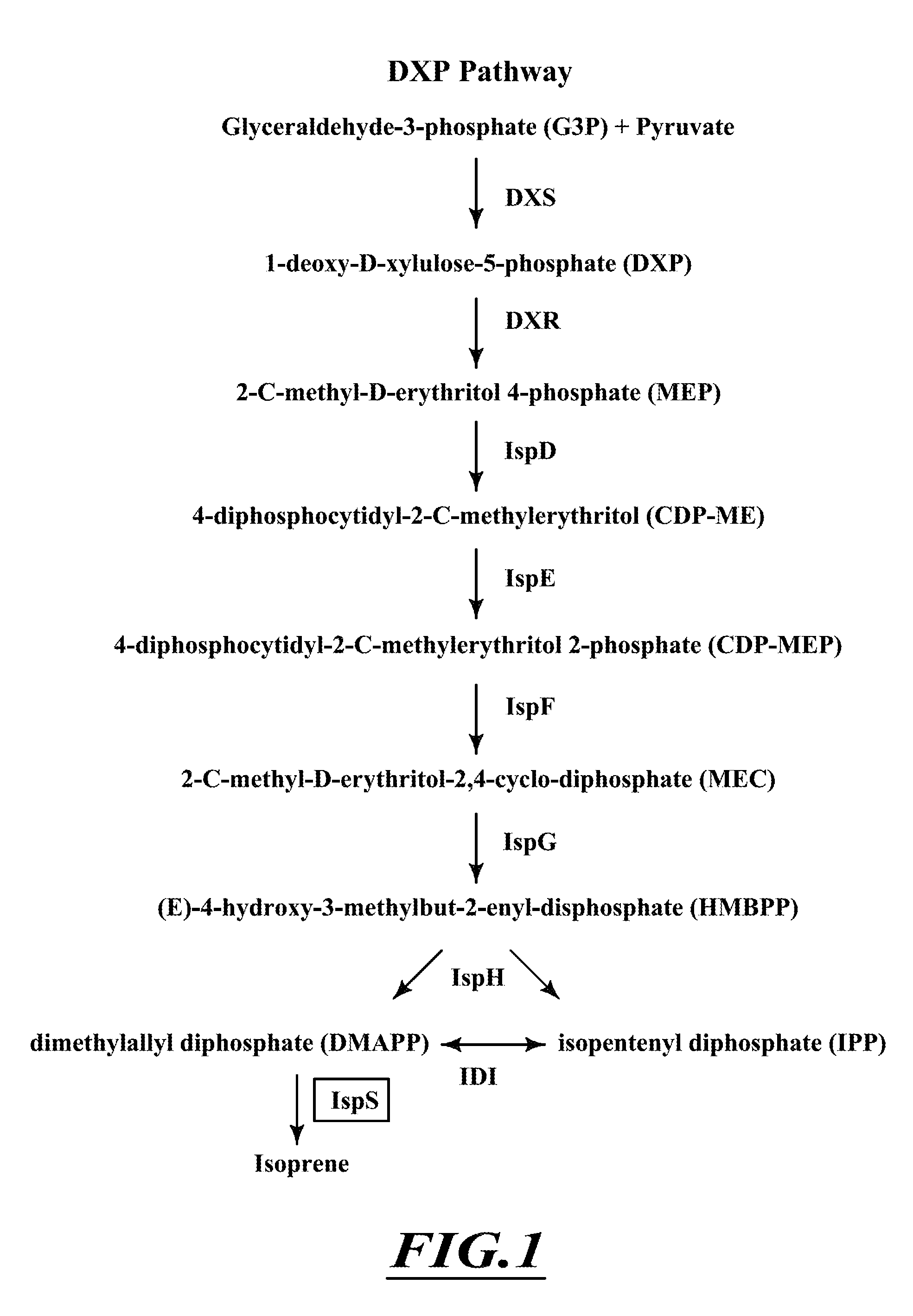
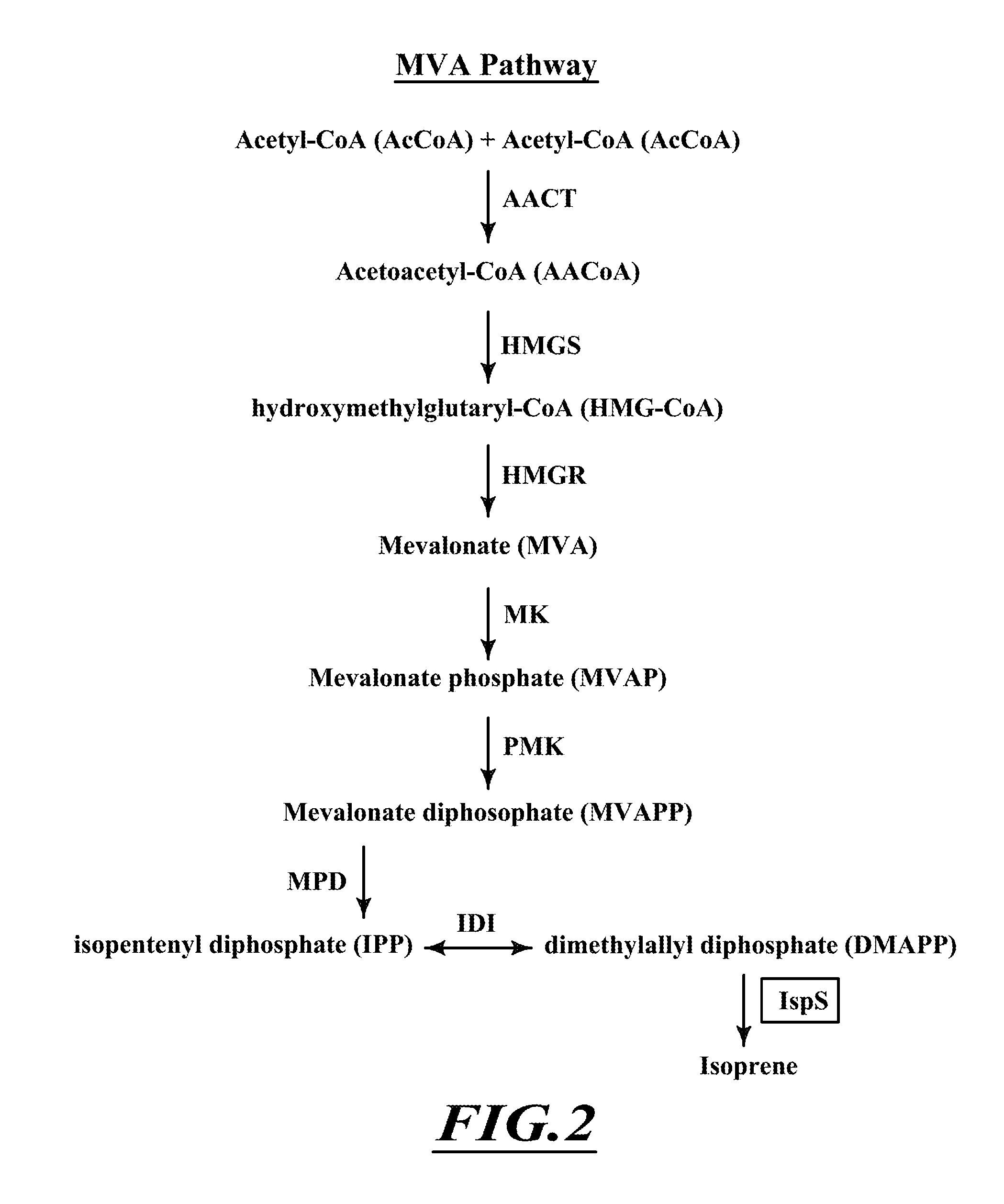
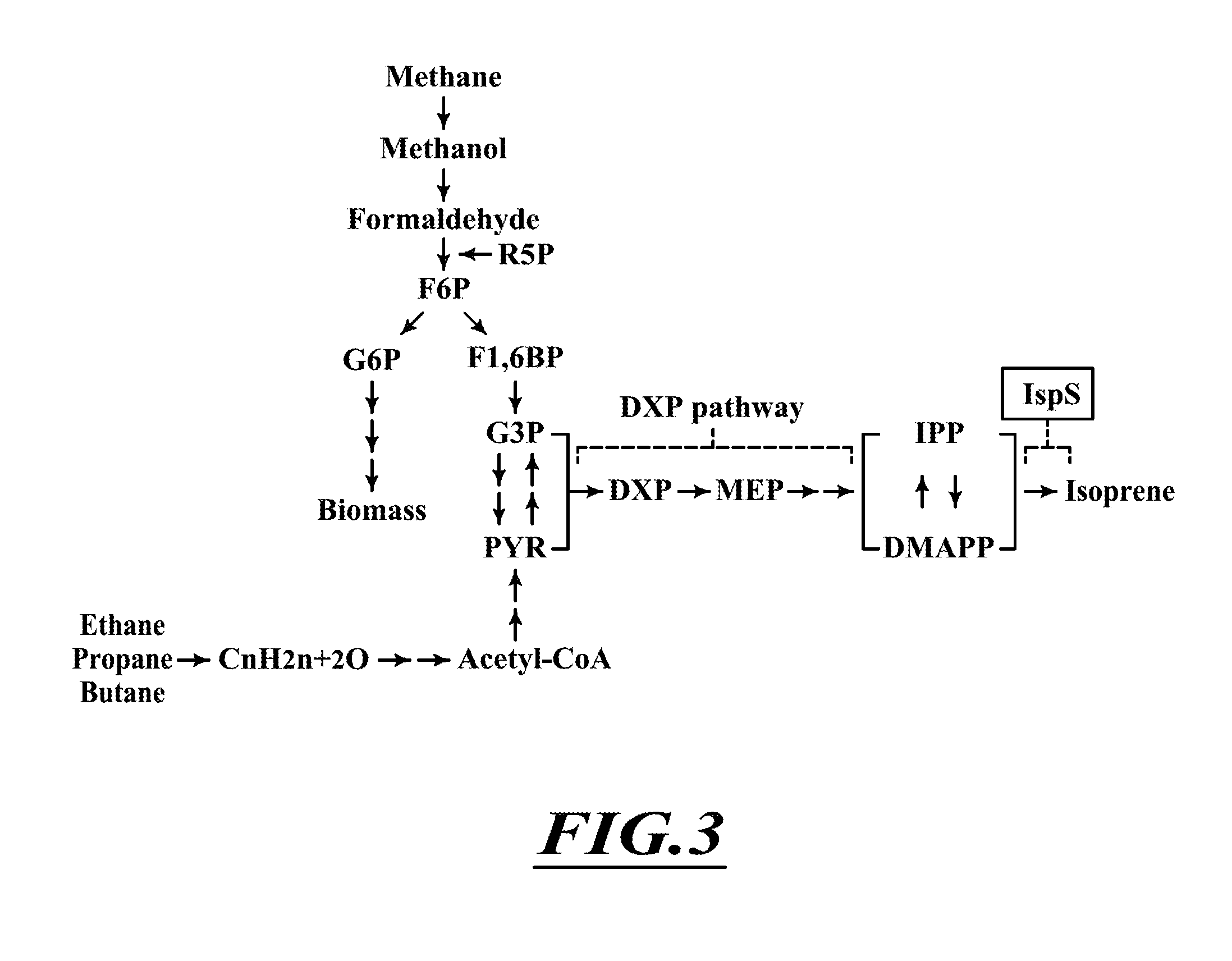
Compositions and methods for biological production of isoprene
a biological and isoprene technology, applied in the direction of lyases, transferases, waste based fuel, etc., can solve the problems of inconvenient insufficient isoprene yields from naturally producing organisms, and limited industrial use of isoprene, so as to increase red pigmentation, increase red pigmentation, and increase the synthesis of isoprene precursors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning and Expression of Isoprene Synthase in Methanococcus Capsulatus Bath Strain
[0124]To create isoprene producing methanotrophic strains, a methanotroph expression vector containing a gene encoding isoprene synthase (IspS) was inserted into the Methylococcus capsulatus Bath, Methylosinus trichosporium OB3b, and Methylomonas sp. 16A via conjugative mating. An episomal expression plasmid (containing sequences encoding origin of replication, origin of transfer, drug resistance marker (kanamycin), and multiple cloning sites), was used to clone either a codon optimized Salix sp. IspS polynucleotide sequence (SEQ ID NO:19 for Methylococcus capsulatus Bath) downstream of a methanol dehydrogenase (MDH) promoter, or a Pueraria montana codon optimized IspS polynucleotide sequence (with the amino-terminal chloroplast targeting sequence removed) (SEQ ID NO:17 for Methylococcus capsulatus Bath) downstream of an IPTG-inducible (LacIq) promoter. Colonies of E. coli strain containing the IspS h...
example 2
Production of Isoprene by Methanococcus Capsulatus Bath Strain
[0126]Headspace gas samples (250 μl) from enclosed 5 mL cultures grown overnight of M. capsulatus Bath strain containing either a vector containing constitutive MDH promoter-Salix sp. IspS or a vector containing an IPTG-inducible (LacIq) promoter-Pueraria montana IspS (grown in the presence or absence of 0.1-10 mM IPTG) were obtained. Gas samples were injected onto a gas chromatograph with flame ionization detector (Hewlett Packard 5890). Chromatography conditions include an Agilent CP-PoraBOND U (25 m×0.32 mm i.d.) column, oven program 50° C., 1.5 min; 25° C., 1 min; 300° C., 10 min. The eluted peak was detected by flame ionization and integrated peaks were quantitated by comparison to isoprene standard (pure isoprene dissolved in deionized water).
[0127]M. capsulatus Bath produced more isoprene when expressing the Pueraria montana IspS as compared to expression of the Salix sp. IspS. In addition, and the amount of isopre...
example 3
Engineering a DXP Pathway with Improved Isoprene Production
[0128]Random mutations are introduced in the DXP pathway operon (i.e., DXS-DXR-IspD-IspE-IspF-IspG-IspH) for the purpose of generating novel gene sequences or regulatory elements within the pathway that overall, result in an improvement of enzymes for synthesis of the committed precursors of isoprene (IPP and DMAPP). To construct a facile high-throughput screening method for isolating an improved DXP pathway, a lycopene synthesis pathway comprising ggpps, crtB and crtI was utilized as a colorimetric reporter. A random mutagenesis library of the DXP pathway is created by error-prone PCR at low, medium, and high mutation rate using GENEMORPH® II random mutagenesis kit (Stratagene). The library is then cloned into a methanotrophic expression plasmid containing ggpps, crtB, and crtI gene sequences, whereby their polycistronic expression is driven by a strong methanotroph promoter sequence (e.g., methanol dehydrogenase promoter)....
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com